|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Protein's strength lies in H-bond cooperation |
|
CAMBRIDGE, Mass. - Researchers in Civil and Environmental Engineering at MIT reveal that the strength of a biological material like spider silk lies in the specific geometric configuration of structural proteins, which have small clusters of weak hydrogen bonds that work cooperatively to resist force and dissipate energy. This structure makes the lightweight natural material as strong as steel, even though the �glue� of hydrogen bonds that hold spider silk together at the molecular level is 100 to 1,000 times weaker than the powerful glue of steel�s metallic bonds or even Kevlar�s covalent bonds. |
|
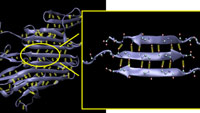
Based on theoretical modeling and large-scale atomistic simulation implemented on supercomputers, this new understanding of exactly how a protein�s configuration enhances a material�s strength could help engineers create new materials that mimic spider silk�s lightweight robustness. It could also impact research on muscle tissue and amyloid fibers found in brain tissue. �Our hope is that by understanding the mechanics of materials at the atomistic level, we will be able to one day create a guiding principle that will direct the synthesis of new materials,� said Professor Markus Buehler, lead researcher on the work. In a paper published in the Feb. 13 online issue of Nano Letters, Buehler and graduate student Sinan Keten describe how they used atomistic modeling to demonstrate that the clusters of three or four hydrogen bonds that bind together stacks of short beta strands in a structural protein rupture simultaneously rather than sequentially when placed under mechanical stress. This allows the protein to withstand more force than if its beta strands had only one or two bonds. Oddly enough, the small clusters also withstand more energy than longer beta strands with many hydrogen bonds. �Using only one or two hydrogen bonds in building a protein provides no or very little mechanical resistance, because the bonds are very weak and break almost without provocation,� said Buehler, the Esther and Harold E. Edgerton Assistant Professor in the Department of Civil and Environmental Engineering. �But using three or four bonds leads to a resistance that actually exceeds that of many metals. Using more than four bonds leads to a much-reduced resistance. The strength is maximized at three or four bonds.� After observing the simultaneous rupture of these hydrogen-bond clusters within the proteins in their atomistic simulations, Buehler and Keten wanted to know why the bonds break in small clusters, even in long strands with many hydrogen bonds. They used the laws of thermodynamics to explain this phenomenon. The paper in Nano Letters describes how the external force changes the entropic energy in the system and leads to the rupture of hydrogen bonds. By calculating the energy necessary to initiate the unfolding process in a protein molecule, they demonstrated that adding more hydrogen bonds in longer strands would not increase the material�s strength. �You would simply have this long chain of beta strands with lazy bonds that don�t contribute to the strength of the assembly,� said Keten. �But a material that employs many short beta strands folded and connected by three or four hydrogen bonds may exhibit strength greater than steel. In metals, the energy would be stored directly in much stronger bonds, called metallic bonds, until bonds rupture one by one. In proteins, things are more complicated due to the entropic elasticity of the noodle-like chains and the cooperative nature of the hydrogen bonds.� Structural proteins contain a preponderance of beta-sheets, sections that fold in such a way that they look a bit like old-fashioned ribbon candy; short waves or strands appear to be stacked on top of one another, each just the right length to allow three or four hydrogen bonds to connect it to the section above and beneath. Beta sheets with short strand lengths connected by three or four hydrogen bonds are the most common conformation among all beta-structured proteins, including those comprising muscle tissue, according to experimental proteomics data on protein structures in the Protein Data Bank. This correlation of a common geometric configuration among beta sheets - which are one of the two most prevalent protein structures in existence - suggests that a protein�s strength is an important evolutionary driving force behind its physical design. The researchers observed the same behavior in similar small clusters in alpha-helical structural proteins, the other most prevalent protein, but haven�t yet studied those assemblies in detail. On the other hand, synthetic materials like steel have a very different and crystalline structure held together by the stronger glue of metallic bonds. Because steel and other synthetic materials tend to be dense, and therefore heavy, they consume a good deal of energy in manufacturing and transport. �Metals are configured with bonds that are much stronger and require a much greater force to break,� said Buehler. �However, the crystalline lattice of a metal�s structure is never perfect; it contains defects that effectively reduce the material�s strength quite drastically. When you place a load on the metal, the defect can fail, possibly causing a crack to propagate. In protein�s beta sheets, the confined nature of the hydrogen bond clusters helps to dissipate the energy without compromising the strength of the material. This shows the amazing ingenuity and efficiency of natural materials.� This research was supported by an MIT Presidential Graduate Fellowship, the Army Research Office, a National Science Foundation CAREER Award, the Solomon Buchsbaum AT&T Research Fund, and a grant from the San Diego Supercomputing Center (SDSC). |
|
|
|
|
Related topics - search form: |
|
|