 |
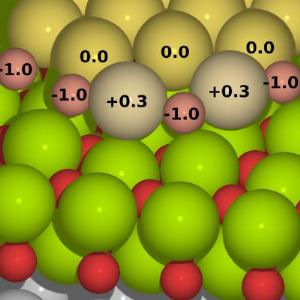
A predicted atomic configuration of the gold-oxide chains (gold: gray, oxygen: pink) at the boundary of a monolayer-thick gold cluster (gold: yellow), supported by a thin magnesium oxide (magnesium: green, oxygen: red) on silver. The numbers indicate atomic charges in units of electron charge.
[Credit: Angewandte Chemie] |
The study was published in September 2010 in Angewandte Chemie, the leading international journal in chemistry. The study is part of Karoliina Honkala's Academy of Finland Academy Researcher project and it was conducted in cooperation with Professor Hannu Häkkinen. The computational work was facilitated by extensive resources from the Finnish IT Center for Science, CSC.
In the study, researchers exposed the monolayer-thick gold clusters to a variable number of oxygen molecules. It was found that even one gold cluster can effectively adsorb multiple oxygen molecules at the boundaries of the cluster, simultaneously weakening (stretching) the O-O bond by transferring electrons to the oxygen molecules. Taking into account the effects of temperature and ambient pressure, the calculations predicted that the oxygen molecules will completely dissociate and the oxygen and gold atoms will form one-dimensional alternating chains at the cluster boundary (see Figure). The oxygen atoms in these chains are negatively and the gold atoms positively charged, creating a system that is reminiscent to a one-dimensional gold-oxide chain. These chains are expected to be the highly catalytically active part towards conversion of carbon monoxide to carbon dioxide at room temperature.
Researchers Pentti Frondelius, Hannu Häkkinen and Karoliina Honkala have studied monolayer-thick gold clusters with 10-20 atoms, supported by thin magnesium oxide films that were grown on silver metal. These systems can be prepared experimentally, and last year the Jyväskylä group published a joint study with Professor Hans-Joachim Freund from the Fritz-Haber Institute in Berlin to characterize atomic and electronic structures of gold clusters in such systems (see below).
Intensive experimental work since the early 1980s has indicated that gold nanoparticles exhibit unexpected catalytic activity towards many industrially important chemical reactions that involve activation of atomic bonds inside oxygen or hydrocarbon molecules. Room-temperature formation of carbon dioxide (CO2) from carbon monoxide (CO) and oxygen molecule (O2) is one of the most extensively studied processes. A number of different factors have been suggested to contribute to the ability of gold particles to activate the O-O bond, which is considered to be the key reaction step.
"The study now published provides us with a new approach to the problem. The formation of gold oxide, that is, the oxidation of gold, is in contradiction with the known properties of macroscopic gold metal. On the nanometer scale, however, everything seems to be possible," Professor Häkkinen says.



