Die 4,5-Dianilinophthalimide - Abkürzung: DAPH - bilden eine Gruppe von organisch-chemischen Verbindungen, die sich von der nebenstehenden Struktur ableiten:
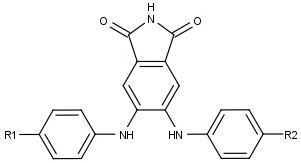
| Dianilinophthalimid | Name | Summenformel | R1 | R2 |
| DAPH-1 | 4,5-Dianilinophthalimid | C20H15N3O2 | H | H |
| DAPH-7 | 4,5-Bis[(4-Fluorophenyl)amino]phthalimid | C20H13F2N3O4 | F | F |
| DAPH-12 | 4,5-Bis-(4-Methoxyanilino)phthalimid | C22H19N3O4 | O-CH3 | O-CH3H |
Quellen und weitere Informationen:
[1] - Uwe Trinks et al.:
Dianilinophthalimides: Potent and Selective, ATP-Competitive Inhibitors of the EGF-Receptor Protein Tyrosine Kinase.
In: J. Med. Chem., (1994), DOI 10.1021/jm00033a019.
[2] - Edward J. Hennessy, Stephen L. Buchwald:
Synthesis of 4,5-Dianilinophthalimide and Related Analogues for Potential Treatment of Alzheimer's Disease via Palladium-Catalyzed Amination.
In: J. Org. Chem., (2005), DOI 10.1021/jo051096o.
[3] - Huan Wang et al.:
Direct and selective elimination of specific prions and amyloids by 4,5-dianilinophthalimide and analogs.
In: PNAS, (2008), DOI 10.1073/pnas.0801934105.
[4] - NN:
Green Tea Chemistry: EGCG and DAPH-12.
In: Internetchemistry News, (2009), DOI https://www.internetchemistry.com/news/2009/dec09/egcg-and-daph-12-in-green-tea.php.
Kategorie: Stoffgruppen
Aktualisiert am 24. Juni 2023.
Permalink: https://www.internetchemie.info/chemie-lexikon/stoffgruppen/d/dianilinophthalimide.php
© 1996 - 2026 Internetchemie ChemLin