|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
New technique captures chemical reactions in a single living cell for the first time |
|
The technique, described in the Nov. 18 issue of the journal Nature Methods, could lead to a new era in molecular imaging with implications for cell-based drug discovery and biomedical diagnostics. The researchers point out that other techniques, such as nuclear magnetic resonance, can at best provide information about a cluster of cells. But to determine the earliest signs of disease progression or of stem cell proliferation, it's necessary to drill down deeper to the molecular dynamics within a single cell. |
|
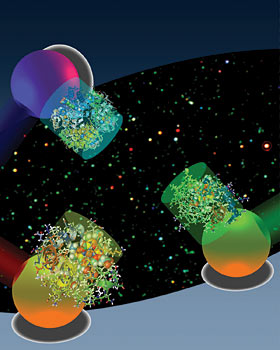
To study the biochemical processes of a cell, scientists currently cut through its outer membrane to separate and analyze the cellular components. That method can never provide a real-time view of how components function together because the cell is killed in the process of extracting its components. "Until now, there has been no non-invasive method that exists that can capture the chemical fingerprints of molecules with nanoscale spatial resolution within a single living cell," said Lee, who is also a faculty affiliate of the California Institute for Quantitative Biosciences and the co-director of the Berkeley Sensor and Actuator Center. "There is great hope that stem cells can one day be used to treat diseases, but one of the biggest challenges in this field is understanding exactly how individual cells differentiate. What is happening inside a stem cell as it develops into a heart muscle instead of a tooth or a strand of hair? To find out, we need to look at the telltale chemical signals involved as proteins and genes function together within a cell." The researchers tackled this challenge by improving upon conventional optical absorption spectroscopy, a technique by which light is passed through a solution of molecules to determine which wavelengths are absorbed. Cytochrome c, for instance, is a protein involved in cell metabolism and cell death that has several optical absorption peaks of around 550 nanometers. The absorption spectra of a molecule can change based upon the chemical changes that occur as it interacts with other molecules, such as oxygen. "For conventional optical absorption spectroscopy to work, a relatively high concentration of biomolecules and a large volume of solution is needed in order to detect these subtle changes in frequencies and absorption peaks," said Lee. "That's because optical absorption signals from a single biomolecule are very weak, so you need to kill hundreds to millions of cells to fish out enough of the target molecule for detection." The researchers came up with a novel solution to this problem by coupling biomolecules, the protein cytochrome c in this study, with tiny particles of gold measuring 20-30 nanometers long. The electrons on the surface of metal particles such as gold and silver are known to oscillate at specific frequencies in response to light, a phenomenon known as plasmon resonance. The resonant frequencies of the gold nanoparticles are much easier to detect than the weak optical signals of cytochrome c, giving the researchers an easier target. Gold nanoparticles were chosen because they have a plasmon resonance wavelength ranging from 530 to 580 nanometers, corresponding to the absorption peak of cytochrome c. "When the absorption peak of the biomolecule overlaps with the plasmon resonance frequency of the gold particle, you can see whether they are exchanging energy," said study co-lead author Gang Logan Liu, who conducted the research as a UC Berkeley Ph.D. student in bioengineering. "This energy transfer shows up as small dips, something we call 'quenching,' in the characteristic absorption peak of the gold particle." A relatively small concentration of the molecule is needed to create these quenching dips, so instead of a concentration of millions of molecules, researchers can get by with hundreds or even dozens of molecules. The sensitivity and selectivity of the quenching dips will improve the molecular diagnosis of diseases and be instrumental in the development of personalized medicine, the researchers said. The researchers repeated the experiment matching the protein hemoglobin with silver nanoparticles and achieved similar results. "Our technique kills two birds with one stone," Lee said. "We're reducing the spatial resolution required to detect the molecule at the same time we're able to obtain chemical information about molecules while they are in a living cell. In a way, these gold particles are like 'nano-stars' because they illuminate the inner life of a cellular galaxy." Other researchers on the UC Berkeley team are Yi-Tao Long, co-lead author and postdoctoral scholar in bioengineering; Yeonho Choi, a Ph.D. student in mechanical engineering; and Taewook Kang, a postdoctoral scholar in bioengineering. The Ministry of Science and Technology in Korea helped support this research. |
|
|
|