|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
|
- Imprint |
|
|
Life at the jolt |
|
Researchers at the Biodesign Institute are using the tiniest organisms on the planet 'bacteria' as a viable option to make electricity. In a new study featured in the journal Biotechnology and Bioengineering, lead author Andrew Kato Marcus and colleagues Cesar Torres and Bruce Rittmann have gained critical insights that may lead to commercialization of a promising microbial fuel cell (MFC) technology. "We can use any kind of waste, such as sewage or pig manure, and the microbial fuel cell will generate electrical energy," said Marcus, a Civil and Environmental Engineering graduate student and a member of the institute's Center for Environmental Biotechnology. Unlike conventional fuel cells that rely on hydrogen gas as a fuel source, the microbial fuel cell can handle a variety of water-based organic fuels. |
|
"There is a lot of biomass out there that we look at simply as energy stored in the wrong place," said Bruce Rittmann, director of the center. "We can take this waste, keeping it in its normal liquid form, but allowing the bacteria to convert the energy value to our society's most useful form, electricity. They get food while we get electricity."
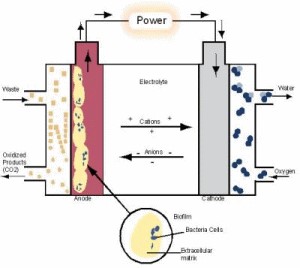
Waste not Bacteria have such a rich diversity that researchers can find a bacterium that can handle almost any waste compound in their daily diet. By linking bacterial metabolism directly with electricity production, the MFC eliminates the extra steps necessary in other fuel cell technologies. "We like to work with bacteria, because bacteria provide a cheap source of electricity," said Marcus. There are many types of MFC reactors and research teams throughout the world (http://microbialfuelcell.org). However, all reactors share the same operating principles. All MFCs have a pair of battery-like terminals: an anode and cathode electrode. The electrodes are connected by an external circuit and an electrolyte solution to help conduct electricity. The difference in voltage between the anode and cathode, along with the electron flow in the circuit, generate electrical power. In the first step of the MFC, an anode respiring bacterium breaks down the organic waste to carbon dioxide and transfers the electrons released to the anode. Next, the electrons travel from the anode, through an external circuit to generate electrical energy. Finally, the electrons complete the circuit by traveling to the cathode, where they are taken up by oxygen and hydrogen ions to form water.
What is the matrix? "We knew that the MFC process is relatively stable, but one of the biggest questions is: How do the bacteria get the electrons to the anode?" said Marcus. The bacteria depend on the anode for life. The bacteria at the anode breathe the anode, much like people breathe air, by transferring electrons to the anode. Because bacteria use the anode in their metabolism, they strategically position themselves on the anode surface to form a bacterial community called a biofilm. Bacteria in the biofilm produce a matrix of material so that they stick to the anode. The biofilm matrix is rich with material that can potentially transport electrons. The sticky biofilm matrix is made up of a complex of extracellular proteins, sugars, and bacterial cells. The matrix also has been shown to contain tiny conductive nanowires that may help facilitate electron conduction. "Our numerical model develops and supports the idea that the bacterial matrix is conductive," said Marcus. In electronics, conductors are most commonly made of materials like copper that make it easier for a current to flow through . "In a conductive matrix, the movement of electrons is driven by the change in the electrical potential." Like a waterfall, the resulting voltage drop in the electrical potential pushes the flow of electrons. The treatment of the biofilm matrix as a conductor allowed the team to describe the transport of electrons driven by the gradient in the electrical potential. The relationship between the biofilm matrix and the anode could now be described by a standard equation for an electrical circuit, Ohm's law. Within the MFC is a complex ecosystem where bacteria are living within a self-generated matrix that conducts the electrons. "The whole biofilm is acting like the anode itself, a living electrode," said Marcus. "This is why we call it the 'biofilm anode.'"
Life at the Jolt The concept of the 'biofilm anode' allowed the team to describe the transport of electrons from bacteria to the electrode and the electrical potential gradient. The importance of electrical potential is well known in a traditional fuel cell, but its relevance to bacterial metabolism has been less clear. The next important concept the team had to develop was to understand the response of bacteria to the electrical potential within the biofilm matrix. Bacteria will grow as long as there is an abundant supply of nutrients. Jacques Monod, one of the founding fathers of molecular biology, developed an equation to describe this relationship. While the team recognized the importance of the Monod equation for bacteria bathed in a rich nutrient broth, the challenge was to apply the Monod equation to the anode, a solid. Previous studies have shown that the rate of bacterial metabolism at the anode increases when the electrical potential of the anode increases. The researchers could now think of the electrical potential as fulfilling the same role as a bacterial nutrient broth. The team recognized that the electrical potential is equivalent to the concentration of electrons; and the electrons are precisely what the bacteria transfer to the anode. Equipped with this key insight, the team developed a new model, the Nernst-Monod equation, to describe the rate of bacterial metabolism in response to the "concentration of electrons" or the electrical potential.
Promise meeting potential In their model, the team identified three crucial variables to controlling an MFC: the amount of waste material (fuel), the accumulation of biomass on the anode, and the electrical potential in the biofilm anode. The third factor is a totally novel concept in MFC research. "Modeling the potential in the biofilm anode, we now have a handle on how the MFC is working and why. We can predict how much voltage we get and how to maximize the power output by tweaking the various factors," said Marcus. For example, the team has shown that the biofilm produces more current when the biofilm thickness is at a happy medium, not too thick or thin. "If the biofilm is too thick," said Marcus, "the electrons have to travel too far to get to the anode. On the other hand, if the biofilm is too thin, it has too few bacteria to extract the electrons rapidly from the fuel." To harvest the benefits of MFCs, the research team is using its innovative model to optimize performance and power output. The project, which has been funded by NASA and industrial partners OpenCEL and NZLegacy, lays out the framework for MFC research and development to pursue commercialization of the technology. |
|
|
|
|
Related topics - search form: |
|
|