|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Discovery of the cell's water gate may lead to new cancer drugs |
|
The flow of water into and out from the cell may play a crucial role in several types of cancer. Scientists at the University of Gothenburg, Sweden, have now found the gate that regulates the flow of water into yeast cells. The discovery, which will be published in the journal PLoS Biology, raises hopes of developing a drug that inhibits the spread and growth of tumours. All living organisms must be able to regulate the flow of water into and out from cells, in order to maintain cell form and size. This regulation is carried out by special proteins known as "aquaporins". These act as water channels and control the flow of water into and out from the cell. |
|
Involved in cancer diseases Aquaporins are found in most organisms, and are believed to be involved in several diseases, including cancer. Research on mice has shown that inhibiting the function of aquaporins can dramatically reduce the spread and growth of tumours.
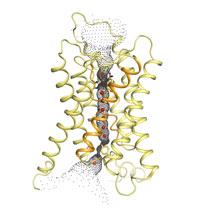
Important for research It is therefore extremely important for cancer research to increase our knowledge of aquaporins. Scientists at the University of Gothenburg have recently achieved a minor breakthrough in the field. Karin Lindkvist at the Department of Cell and Molecular Biology and Richard Neutze at the Department of Chemistry, University of Gothenburg have determined the three-dimensional structure of the yeast aquaporin. The results will be published in the journal PLoS Biology.
Highest resolution The structure has been determined using X-ray crystallography and is the highest resolution structure that has been determined for a membrane protein. The unique high resolution has enabled the scientists to answer one of the unsolved mysteries of biology. The aquaporins in yeast have long "tails", known as amino-terminal extensions. The function of these tails has, until now, been unknown. "Our study shows that the amino-terminal extensions in yeast act as a gate that can be opened and closed depending on how much water the cell must release or absorb. Computer simulations and biological experiments suggest that the channel is regulated with a combination of mechanical regulation and phosphorylation", says Karin Lindkvist.
Similar to human Yeast cells are similar to human cells in many respects, and Karin Lindkvist's research can have applications in cancer research and other fields. "The structure of the yeast aquaporin that we have determined can be used to create inhibitors for human aquaporins, and this may in the long term lead to drugs that slow the growth of a cancer tumour", says Karin Lindkvist. |
|
|
|