|
Related Topics: |
|
|
|
Current News |
|
Chemistry A to Z |
|
About Internetchemistry |
Oxygen in Place of Chlorine |
|
Propylene oxide is an important bulk chemical that is used primarily in the production of polyurethane plastics. Currently, propylene oxide is usually made from propylene (propene) in a process that uses chlorine as an oxidizing agent. This results in undesired byproducts as well as toxic chlorinated organic compounds. Existing alternative routes are mostly complicated and uneconomical. The development of an environmentally friendly propylene oxide synthesis with oxygen as the oxidizing agent is high on the wish list. Japanese researchers have now developed a new catalyst that brings this goal closer. As the scientists working with Masatake Haruta report in the journal Angewandte Chemie, the catalyst is based on gold clusters and a special titanium-containing support. |
|
|
In the oxidation of propylene (propene, CH3–CH=CH2) to propylene oxide (propene oxide), an oxygen atom is formally inserted into the double bond. This forms a ring containing two carbon atoms and one oxygen atom. Using oxygen as the oxidizing agent had not been considered before because the oxygen molecule (O2) can only be split into individual oxygen atoms with the input of a large amount of energy. Furthermore, propylene preferentially reacts with atomic oxygen to form acrolein and not the desired propylene oxide. A suitable catalyst is eagerly sought, and has come to be viewed as the “holy grail” of catalyst research. There have been a number of catalytic developments that have been not quite satisfactory.
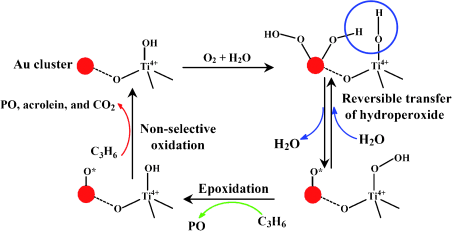
Building upon prior work, Haruta and his team have been able to achieve a further step. Their new catalyst consists of gold clusters, which are less than 2 nm in size, deposited on a special titanium-containing silicalite support. “It is important that the gold used is not in the form of nanoparticles, but is in clusters,” emphasizes Haruta. Although these two terms are often used interchangeably in the literature, there are important differences. Gold clusters are explicitly defined, structurally uniform nanoscopic structures, whereas gold nanoparticles are particles with size in the nanometer range that have neither uniform size nor structure. “Our gold clusters are able to convert oxygen and water into hydroperoxide species, which are transferred onto neighboring titanium centers,” explains Haruta. “The resulting titanium hydroperoxide species (Ti–OOH) are the actual reaction partners for the propylene, which is converted to propylene oxide.” “The yields and selectivities we have achieved so far are inadequate for an industrial process,” says Haruta, “however, our catalyst is another important milestone on the way to an environmentally friendly synthesis for propylene oxide.” |
|
|
|