Unter Erbium-Isotope werden alle Atomkerne des chemischen Elements Erbium zusammengefasst; diese bestehen allesamt aus einem Atomkern mit 68 Protonen und im ungeladenen Zustand aus 68 Elektronen. Der Unterschied zwischen den einzelnen Erbium-Isotopen beruht auf der Anzahl der Neutronen im Kern.
Natürlich auftretende Erbium-Isotope
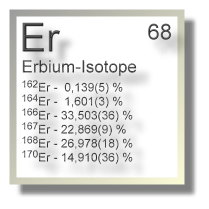
Natürliche irdische Erbium-Vorkommen bestehen aus einem Gemisch aus sechs verschiedenen stabilen Isotopen:
| Atommasse Ar | Anteil | Halbwertszeit | Spin | |
| Erbium Isotopengemisch | 167,259 u | 100 % | ||
| Isotop 162Er | 161,92879(2) u | 0,139(5) % | stabil | 0+ |
| Isotop 164Er | 163,92921(2) u | 1,601(3) % | stabil | 0+ |
| Isotop 166Er | 165,93030(2) u | 33,503(36) % | stabil | 0+ |
| Isotop 167Er | 166,93205(2) u | 22,869(9) % | stabil | 7/2+ |
| Isotop 168Er | 167,93238(2) u | 26,978(18) % | stabil | 0+ |
| Isotop 170Er | 169,93547(2) u | 14,910(36) % | stabil | 0+ |
Alle anderen bisher bekannten und charakterisierten Erbium-Nuklide sind instabil und besitzen keinerlei Bedeutung für technische Anwendungen.
Isotopentabelle: Erbium
| Isotop Nuklid | Z | A | N | Name | Atommasse [Kernmasse] {Massenüberschuss} | Spin I (h/2π) | μ | A-Nuk |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 143Er | 68 | 143 | 75 | Erbium-143 | 142,96644(43) u [142,9291399 u] {-31,26094 MeV} | 0+ | ||
| 144Er | 68 | 144 | 76 | Erbium-144 | 143,96070(21) u [143,9233999 u] {-36,60771 MeV} | 0+ | 145Tm | |
| 145Er | 68 | 145 | 77 | Erbium-145 | 144,95787(21) u [144,9205699 u] {-39,24384 MeV} | (1/2+) | 146Tm | |
| 145mEr | 68 | 145 | 77 | Erbium-145m | 144,95787(21) u [144,9205699 u] {-39,24384 MeV} | (11/2-) | ||
| 146Er | 68 | 146 | 78 | Erbium-146 | 145,952418(7) u [145,9151179 u] {-44,32235 MeV} | 0+ | 147Tm | |
| 147Er | 68 | 147 | 79 | Erbium-147 | 146,94996(4) u [146,9126599 u] {-46,61196 MeV} | (1/2+) | 147Tm | |
| 147mEr | 68 | 147 | 79 | Erbium-147m | 146,94996(4) u [146,9126599 u] {-46,61196 MeV} | (11/2-) | ||
| 148Er | 68 | 148 | 80 | Erbium-148 | 147,944735(11) u [147,9074349 u] {-51,47902 MeV} | 0+ | ||
| 148mEr | 68 | 148 | 80 | Erbium-148m | 147,944735(11) u [147,9074349 u] {-51,47902 MeV} | (10+) | ||
| 149Er | 68 | 149 | 81 | Erbium-149 | 148,94231(3) u [148,9050099 u] {-53,73789 MeV} | (1/2+) | 149Tm | |
| 149m1Er | 68 | 149 | 81 | Erbium-149m1 | 148,94231(3) u [148,9050099 u] {-53,73789 MeV} | (11/2-) | ||
| 150Er | 68 | 150 | 82 | Erbium-150 | 149,937916(18) u [149,9006159 u] {-57,83087 MeV} | 0+ | 150Tm 151Yb 154Yb | |
| 151Er | 68 | 151 | 83 | Erbium-151 | 150,937449(18) u [150,9001489 u] {-58,26588 MeV} | (7/2-) | 151Tm 155Yb | |
| 151mEr | 68 | 151 | 83 | Erbium-151m | 150,937449(18) u [150,9001489 u] {-58,26588 MeV} | (27/2-) | ||
| 152Er | 68 | 152 | 84 | Erbium-152 | 151,935050(9) u [151,8977499 u] {-60,50054 MeV} | 0+ | 152Tm 153Yb 156Yb | |
| 153Er | 68 | 153 | 85 | Erbium-153 | 152,935084(10) u [152,8977839 u] {-60,46886 MeV} | (7/2-) | - 0,934(5) | 153Tm 157Yb |
| 154Er | 68 | 154 | 86 | Erbium-154 | 153,932791(5) u [153,8954909 u] {-62,60478 MeV} | 0+ | 154Tm 158Yb | |
| 155Er | 68 | 155 | 87 | Erbium-155 | 154,933216(7) u [154,8959159 u] {-62,2089 MeV} | 7/2- | - 0,669(4) | 155Tm |
| 156Er | 68 | 156 | 88 | Erbium-156 | 155,931066(26) u [155,8937659 u] {-64,21161 MeV} | 0+ | 156Tm | |
| 157Er | 68 | 157 | 89 | Erbium-157 | 156,931923(28) u [156,8946229 u] {-63,41332 MeV} | 3/2- | - 0,412(3) | 157Tm |
| 158Er | 68 | 158 | 90 | Erbium-158 | 157,929893(27) u [157,8925929 u] {-65,30425 MeV} | 0+ | 158Tm | |
| 159Er | 68 | 159 | 91 | Erbium-159 | 158,930691(4) u [158,8933909 u] {-64,56092 MeV} | 3/2- | - 0,304(2) | 159Tm |
| 160Er | 68 | 160 | 92 | Erbium-160 | 159,929077(26) u [159,8917769 u] {-66,06435 MeV} | 0+ | 160Tm | |
| 161Er | 68 | 161 | 93 | Erbium-161 | 160,930003(9) u [160,8927029 u] {-65,20179 MeV} | 3/2- | - 0,365(3) | 161Tm |
| 161mEr | 68 | 161 | 93 | Erbium-161m | 160,930003(9) u [160,8927029 u] {-65,20179 MeV} | 11/2- | ||
| 162Er | 68 | 162 | 94 | Erbium-162 | 161,92879(2) u [161,8914899 u] {-66,33169 MeV} | 0+ | 162Tm | |
| 163Er | 68 | 163 | 95 | Erbium-163 | 162,930040(5) u [162,8927399 u] {-65,16732 MeV} | 5/2- | + 0,557(4) | 163Tm |
| 164Er | 68 | 164 | 96 | Erbium-164 | 163,92921(2) u [163,8919099 u] {-65,94046 MeV} | 0+ | 164Tm 164Ho | |
| 165Er | 68 | 165 | 97 | Erbium-165 | 164,9307332(10) u [164,8934331 u] {-64,52161 MeV} | 5/2- | + 0,643(3) | 165Tm |
| 166Er | 68 | 166 | 98 | Erbium-166 | 165,93030(2) u [165,8929999 u] {-64,92513 MeV} | 0+ | 166Tm 166Ho | |
| 167Er | 68 | 167 | 99 | Erbium-167 | 166,93205(2) u [166,8947499 u] {-63,29502 MeV} | 7/2+ | - 0,56385(12) | 167Tm 167Ho |
| 167mEr | 68 | 167 | 99 | Erbium-167m | 166,93205(2) u [166,8947499 u] {-63,29502 MeV} | 1/2- | ||
| 168Er | 68 | 168 | 100 | Erbium-168 | 167,93238(2) u [167,8950799 u] {-62,98762 MeV} | 0+ | 168Tm 168Ho | |
| 169Er | 68 | 169 | 101 | Erbium-169 | 168,9345984(3) u [168,8972983 u] {-60,9212 MeV} | 1/2- | + 0,515(25) | 169Ho |
| 170Er | 68 | 170 | 102 | Erbium-170 | 169,93547(2) u [169,8981699 u] {-60,10931 MeV} | 0+ | 170Ho 170Tm | |
| 171Er | 68 | 171 | 103 | Erbium-171 | 170,9380361(17) u [170,900736 u] {-57,719 MeV} | 5/2- | 0,659(10) | 171Ho |
| 172Er | 68 | 172 | 104 | Erbium-172 | 171,939362(4) u [171,9020619 u] {-56,48393 MeV} | 0+ | 172Ho | |
| 173Er | 68 | 173 | 105 | Erbium-173 | 172,94240(21) u [172,9050999 u] {-53,65405 MeV} | (7/2-) | ||
| 174Er | 68 | 174 | 106 | Erbium-174 | 173,94423(32) u [173,9069299 u] {-51,94942 MeV} | 0+ | ||
| 175Er | 68 | 175 | 107 | Erbium-175 | 174,94777(43) u [174,9104699 u] {-48,65193 MeV} | (9/2+) | ||
| 176Er | 68 | 176 | 108 | Erbium-176 | 175,94994(43) u [175,9126399 u] {-46,63059 MeV} | 0+ | ||
| 177Er | 68 | 177 | 109 | Erbium-177 | 176,95399(54) u [176,9166899 u] {-42,85804 MeV} | (1/2-) |
| Isotop | Zerfall (radioaktiver Zerfall) | AE | Mehr | |||
|---|---|---|---|---|---|---|
| Halbwertszeit | Zerfallsart | Anteil | Energie | Info | ||
| 1 | 10 | 11 | 12 | 13 | 14 | 15 |
| Er-143 | AL | |||||
| Er-144 | 200 ns | EE/β+ zu 144Ho | 100 % | 7,88 MeV | AL | |
| Er-145 | AL | |||||
| Er-145m | 0,9(3) s | EE/β+ zu 145Ho EE, p zu 144Dy | 100 % ? | 253 keV | ||
| Er-146 | 1,7(6) s | EE/β+ zu 146Ho | 100 % | 6,916(9) MeV | AL | |
| Er-147 | ca. 2,5 s | EE/β+ zu 147Ho β+, p zu 146Dy | 100 % > 0 % | 9,15(4) MeV | AL | |
| Er-147m | 2,5(2) s | EE/β+ zu 147Ho β+, p zu 146Dy | ca. 100 % > 0 % | +x keV | ||
| Er-148 | 4,6(2) s | EE/β+ zu 148Ho EE, p zu 147Dy | ca. 99,85 % ca. 0,15 % | 6,51(8) MeV | AL | |
| Er-148m | 13(3) μs | Iso zu 148Er | 100 % | 2913,2(4) keV | ||
| Er-149 | 4(2) s | EE/β+ zu 149Ho EE, p zu 148Dy | 93 % 7 % | 7,90(3) MeV | AL | |
| Er-149m1 | 8,9(2) s | EE/β+ zu 149Ho EE, p zu 148Dy Iso zu 149Er | 96,5(7) % 0,18(7) % 3,5(7) % | 741,8(2) keV | ||
| Er-150 | 18,5(7) s | EE/β+ zu 150Ho | 100 % | 4,115(22) MeV | AL | |
| Er-151 | 23,5(20) s | EE/β+ zu 151Ho | 100 % | 5,356(18) MeV | AL | |
| Er-151m | 0,58(2) s | EE/β+ zu 151Ho Iso zu 151Er | 4,7(4) % 95,3(4) % | 2585,0(5) keV | ||
| Er-152 | 10,3(1) s | EE/β+ zu 152Ho α zu 148Dy | 10(4) % 90(4) % | 3,104(15) MeV 4,9343(16) MeV | AL | |
| Er-153 | 37,1(2) s | EE/β+ zu 153Ho α zu 149Dy | 47(3) % 53(3) % | 4,543(11) MeV 4,8024(14) MeV | AL | |
| Er-154 | 3,73(9) Minuten | EE/β+ zu 154Ho α zu 150Dy | 99,53(13) % 0,47(13) % | 2,034(10) MeV 4,2797(26) MeV | AL | |
| Er-155 | 5,3(3) Minuten | EE/β+ zu 155Ho α zu 151Dy | 99,978(7) % 0,022(7) % | 3,830(18) MeV 4,118(5) MeV | AL | |
| Er-156 | 19,5(10) Minuten | EE/β+ zu 156Ho α zu 152Dy | ca. 100 % Spuren | 1,27(6) MeV 3,481(25) MeV | AL | |
| Er-157 | 18,65(10) Minuten | EE/β+ zu 157Ho | ca. 100 % | 3,42(4) MeV | AL | |
| Er-158 | 2,29(6) Stunden | EE zu 158Ho | 100 % | 0,88(4) MeV | AL | |
| Er-159 | 36(1) Minuten | EE/β+ zu 159Ho | 100 % | 2,768(5) MeV | AL | |
| Er-160 | 28,58(9) Stunden | EE zu 160Ho | 100 % | 0,319(29) MeV | AL | |
| Er-161 | 3,21(3) Stunden | EE/β+ zu 161Ho | 100 % | 1,996(9) MeV | AL | |
| Er-161m | 7,5(7) μs | Iso zu 161Er | 100 % | 396,44(4) keV | ||
| Er-162 | stabil | AL | ||||
| Er-163 | 75,0(4) Minuten | EE/β+ zu 163Ho | 100 % | 1,211(5) MeV | AL | |
| Er-164 | stabil | AL | ||||
| Er-165 | 10,36(4) Stunden | EE zu 165Ho | 100 % | 0,3774(14) MeV | AL | |
| Er-166 | stabil | AL | ||||
| Er-167 | stabil | AL | ||||
| Er-167m | 2,269(6) s | Iso zu 167Er | 100 % | 207,801(5) keV | ||
| Er-168 | stabil | AL | ||||
| Er-169 | 9,392(18) Tage | β- zu 169Tm | 100 % | 0,3535(8) MeV | AL | |
| Er-170 | stabil | AL | ||||
| Er-171 | 7,516(2) Stunden | β- zu 171Tm | 100 % | 1,4913(13) MeV | AL | |
| Er-172 | 49,3(3) Stunden | β- zu 172Tm | 100 % | 0,891(5) MeV | AL | |
| Er-173 | 1,4(1) Minuten | β- zu 173Tm | 100 % | 2,6(2) MeV | AL | |
| Er-174 | 3,2(2) Minuten | β- zu 174Tm | 100 % | 1,91(30) MeV | AL | |
| Er-175 | 1,2(3) Minuten | β- zu 175Tm | 100 % | 3,66(40) MeV | AL | |
| Er-176 | 160 ns | β- zu 176Tm | 100 % | 2,74(41) MeV | AL | |
| Er-177 | 3 s | β- zu 177Tm | 100 % | 4,61(58) MeV | AL | |
Erläuterungen zu den einzelnen Spalten:
1 - Symbol mit Nukleonenzahl.
2 - Z = Anzahl der Protonen (Ordnungszahl).
3 - Massenzahl A.
4 - N = Anzahl der Neutronen.
5 - Bezeichnung des Erbium-Isotops; gegebenenfalls Trivialnamen.
6 - Relative Atommasse des Erbium-Isotops (Isotopenmasse inklusive Elektronen) und in eckigen Klammern die Masse des Atomkerns (Kernmasse, Nuklidmasse ohne Elektronen), jeweils bezogen auf 12C = 12,00000 [2]. Zusätzlich ist der Massenüberschuss (Massenexzess) in MeV angegeben.
7 - Kernspin I, Einheit: h/2π.
8 - Kernmagnetisches Moment μmag.
9 - Ausgangsnuklide: Mögliche, angenommene oder tatsächliche Ausgangs-Nuklide (Mutternuklide, Elternnuklide). Die entsprechenden Zerfalls-Modi sind gegebenenfalls bei den Daten des jeweiligen Ausgangsnuklids zu finden.
10 - Zerfall: Halbwertszeiten des Erbium-Isotops mit a = Jahre; ; d = Tage; h = Stunden; min = Minuten; s = Sekunden.
11 - Zerfall: Zerfallsart in die jeweiligen Tochternuklide mit n = Neutronenemission; p = Protonenemission; α = Alpha-Zerfall; ß- = Beta-Minus-Zerfall unter Elektronenemission; EE = Elektroneneinfang; ß+ = Positronenemission; ε = ß+ und/oder EE; Iso = Isomerieübergang; CZ = Cluster-Zerfall; SZ = Spontanzerfall.
12 - Zerfall: Zerfallsanteil in Prozent (%).
13 - Zerfall: Zerfallsenergie; Partikelenergie bezogen auf Zerfallsart.
14 - AE = Anregungsenergie für metastabile Kerne.
15 - Sonstige Informationen und Hinweise: AL = Weitere Niveaus, so genannte Adopted Levels (Verlinkung auf externe Daten [1]).
Sonstige:
()- Eingeklammerte Ziffern: Unsicherheit zur Darstellung der Streubreite des angegebenen Wertes.
~ - Theoretische Werte oder systematische Trends.
- ungelistet-: Nuklide, die in der Literatur bereits erwänhnt wurden, aber aus irgendwelchen Gründen in den aktuellen Nuklidtabellen nicht mehr zu finden sind, weil sich deren Entdeckung z. B. nicht bestätigt hat.
NMR-aktive Erbium-Nuklide
| Nuklid Anteil Spin I | Kernmagnetisches Moment μ/μN | Gyromagnetisches Verhältnis 107 rad T-1 s-1 | Quadrupol- Moment Q [barn] | Resonanz- Frequenz v0 bei 1 T | Relative Empfindlichkeit H0 = const. v0 = const. * |
|---|---|---|---|---|---|
| 167Er 22,869(9) % 7/2+ | - 0,56385(12) | - 0,7752 | +3,565(29) | 1,2281 | 0,00050 0,6057 |
*) bezogen auf 1H = 1,000
Strahlenschutz
Für den Umgang mit den Erbium-Radionukliden gelten gemäß Strahlenschutzverordnung (StrlSchV 2018) unter anderem folgende Werte (Spalten 1 bis 7):
| Nuklid | Freigrenzen | HRQ-Schwelle | OFK | Tochternuklide | Halbwertszeit | |
|---|---|---|---|---|---|---|
| Er-161+ | 106 Bq | 10 Bq/g | 3,2 Stunden | |||
| Er-165 | 107 Bq | 1000 Bq/g | 10,4 Stunden | |||
| Er-169 | 107 Bq | 1000 Bq/g | 20 TBq | 100 Bq cm-2 | 9,4 Tage | |
| Er-171 | 106 Bq | 100 Bq/g | 0,2 TBq | 10 Bq cm-2 | 7,5 Stunden | |
| Er-172 | 106 Bq | 100 Bq/g | 49,3 Stunden | |||
(HRQ = Hochradioaktive Quellen; OFK = Oberflächenkontamination)
Kernisobare Nuklide des Erbiums
Zu den Erbium-Nukliden isobare Atomkerne befinden sich in der jeweiligen Tabellenzeile; Z = Ordnungszahl; A = Nukleonenzahl (Massenzahl).
| Z: | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Te | I | Xe | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl |
| 143 | 143Te | 143I | 143Xe | 143Cs | 143Ba | 143La | 143Ce | 143Pr | 143Nd | 143Pm | 143Sm | 143Eu | 143Gd | 143Tb | 143Dy | 143Ho | 143Er | |||||||||||||
| 144 | 144I | 144Xe | 144Cs | 144Ba | 144La | 144Ce | 144Pr | 144Nd | 144Pm | 144Sm | 144Eu | 144Gd | 144Tb | 144Dy | 144Ho | 144Er | 144Tm | |||||||||||||
| 145 | 145I | 145Xe | 145Cs | 145Ba | 145La | 145Ce | 145Pr | 145Nd | 145Pm | 145Sm | 145Eu | 145Gd | 145Tb | 145Dy | 145Ho | 145Er | 145Tm | |||||||||||||
| 146 | 146Xe | 146Cs | 146Ba | 146La | 146Ce | 146Pr | 146Nd | 146Pm | 146Sm | 146Eu | 146Gd | 146Tb | 146Dy | 146Ho | 146Er | 146Tm | ||||||||||||||
| 147 | 147Xe | 147Cs | 147Ba | 147La | 147Ce | 147Pr | 147Nd | 147Pm | 147Sm | 147Eu | 147Gd | 147Tb | 147Dy | 147Ho | 147Er | 147Tm | ||||||||||||||
| 148 | 148Xe | 148Cs | 148Ba | 148La | 148Ce | 148Pr | 148Nd | 148Pm | 148Sm | 148Eu | 148Gd | 148Tb | 148Dy | 148Ho | 148Er | 148Tm | 148Yb | |||||||||||||
| 149 | 149Cs | 149Ba | 149La | 149Ce | 149Pr | 149Nd | 149Pm | 149Sm | 149Eu | 149Gd | 149Tb | 149Dy | 149Ho | 149Er | 149Tm | 149Yb | ||||||||||||||
| 150 | 150Cs | 150Ba | 150La | 150Ce | 150Pr | 150Nd | 150Pm | 150Sm | 150Eu | 150Gd | 150Tb | 150Dy | 150Ho | 150Er | 150Tm | 150Yb | 150Lu | |||||||||||||
| 151 | 151Cs | 151Ba | 151La | 151Ce | 151Pr | 151Nd | 151Pm | 151Sm | 151Eu | 151Gd | 151Tb | 151Dy | 151Ho | 151Er | 151Tm | 151Yb | 151Lu | |||||||||||||
| 152 | 152Ba | 152La | 152Ce | 152Pr | 152Nd | 152Pm | 152Sm | 152Eu | 152Gd | 152Tb | 152Dy | 152Ho | 152Er | 152Tm | 152Yb | 152Lu | ||||||||||||||
| 153 | 153Ba | 153La | 153Ce | 153Pr | 153Nd | 153Pm | 153Sm | 153Eu | 153Gd | 153Tb | 153Dy | 153Ho | 153Er | 153Tm | 153Yb | 153Lu | 153Hf | |||||||||||||
| 154 | 154La | 154Ce | 154Pr | 154Nd | 154Pm | 154Sm | 154Eu | 154Gd | 154Tb | 154Dy | 154Ho | 154Er | 154Tm | 154Yb | 154Lu | 154Hf | ||||||||||||||
| 155 | 155La | 155Ce | 155Pr | 155Nd | 155Pm | 155Sm | 155Eu | 155Gd | 155Tb | 155Dy | 155Ho | 155Er | 155Tm | 155Yb | 155Lu | 155Hf | 155Ta | |||||||||||||
| 156 | 156Ce | 156Pr | 156Nd | 156Pm | 156Sm | 156Eu | 156Gd | 156Tb | 156Dy | 156Ho | 156Er | 156Tm | 156Yb | 156Lu | 156Hf | 156Ta | ||||||||||||||
| 157 | 157Ce | 157Pr | 157Nd | 157Pm | 157Sm | 157Eu | 157Gd | 157Tb | 157Dy | 157Ho | 157Er | 157Tm | 157Yb | 157Lu | 157Hf | 157Ta | ||||||||||||||
| 158 | 158Pr | 158Nd | 158Pm | 158Sm | 158Eu | 158Gd | 158Tb | 158Dy | 158Ho | 158Er | 158Tm | 158Yb | 158Lu | 158Hf | 158Ta | 158W | ||||||||||||||
| 159 | 159Pr | 159Nd | 159Pm | 159Sm | 159Eu | 159Gd | 159Tb | 159Dy | 159Ho | 159Er | 159Tm | 159Yb | 159Lu | 159Hf | 159Ta | 159W | 159Re | |||||||||||||
| 160 | 160Nd | 160Pm | 160Sm | 160Eu | 160Gd | 160Tb | 160Dy | 160Ho | 160Er | 160Tm | 160Yb | 160Lu | 160Hf | 160Ta | 160W | 160Re | ||||||||||||||
| 161 | 161Nd | 161Pm | 161Sm | 161Eu | 161Gd | 161Tb | 161Dy | 161Ho | 161Er | 161Tm | 161Yb | 161Lu | 161Hf | 161Ta | 161W | 161Re | 161Os | |||||||||||||
| 162 | 162Pm | 162Sm | 162Eu | 162Gd | 162Tb | 162Dy | 162Ho | 162Er | 162Tm | 162Yb | 162Lu | 162Hf | 162Ta | 162W | 162Re | 162Os | ||||||||||||||
| 163 | 163Pm | 163Sm | 163Eu | 163Gd | 163Tb | 163Dy | 163Ho | 163Er | 163Tm | 163Yb | 163Lu | 163Hf | 163Ta | 163W | 163Re | 163Os | ||||||||||||||
| 164 | 164Sm | 164Eu | 164Gd | 164Tb | 164Dy | 164Ho | 164Er | 164Tm | 164Yb | 164Lu | 164Hf | 164Ta | 164W | 164Re | 164Os | 164Ir | ||||||||||||||
| 165 | 165Sm | 165Eu | 165Gd | 165Tb | 165Dy | 165Ho | 165Er | 165Tm | 165Yb | 165Lu | 165Hf | 165Ta | 165W | 165Re | 165Os | 165Ir | ||||||||||||||
| 166 | 166Eu | 166Gd | 166Tb | 166Dy | 166Ho | 166Er | 166Tm | 166Yb | 166Lu | 166Hf | 166Ta | 166W | 166Re | 166Os | 166Ir | 166Pt | ||||||||||||||
| 167 | 167Gd | 167Tb | 167Dy | 167Ho | 167Er | 167Tm | 167Yb | 167Lu | 167Hf | 167Ta | 167W | 167Re | 167Os | 167Ir | 167Pt | |||||||||||||||
| 168 | 168Gd | 168Tb | 168Dy | 168Ho | 168Er | 168Tm | 168Yb | 168Lu | 168Hf | 168Ta | 168W | 168Re | 168Os | 168Ir | 168Pt | |||||||||||||||
| 169 | 169Gd | 169Tb | 169Dy | 169Ho | 169Er | 169Tm | 169Yb | 169Lu | 169Hf | 169Ta | 169W | 169Re | 169Os | 169Ir | 169Pt | |||||||||||||||
| 170 | 170Tb | 170Dy | 170Ho | 170Er | 170Tm | 170Yb | 170Lu | 170Hf | 170Ta | 170W | 170Re | 170Os | 170Ir | 170Pt | 170Au | |||||||||||||||
| 171 | 171Tb | 171Dy | 171Ho | 171Er | 171Tm | 171Yb | 171Lu | 171Hf | 171Ta | 171W | 171Re | 171Os | 171Ir | 171Pt | 171Au | 171Hg | ||||||||||||||
| 172 | 172Dy | 172Ho | 172Er | 172Tm | 172Yb | 172Lu | 172Hf | 172Ta | 172W | 172Re | 172Os | 172Ir | 172Pt | 172Au | 172Hg | |||||||||||||||
| 173 | 173Dy | 173Ho | 173Er | 173Tm | 173Yb | 173Lu | 173Hf | 173Ta | 173W | 173Re | 173Os | 173Ir | 173Pt | 173Au | 173Hg | |||||||||||||||
| 174 | 174Ho | 174Er | 174Tm | 174Yb | 174Lu | 174Hf | 174Ta | 174W | 174Re | 174Os | 174Ir | 174Pt | 174Au | 174Hg | ||||||||||||||||
| 175 | 175Ho | 175Er | 175Tm | 175Yb | 175Lu | 175Hf | 175Ta | 175W | 175Re | 175Os | 175Ir | 175Pt | 175Au | 175Hg | ||||||||||||||||
| 176 | 176Er | 176Tm | 176Yb | 176Lu | 176Hf | 176Ta | 176W | 176Re | 176Os | 176Ir | 176Pt | 176Au | 176Hg | 176Tl | ||||||||||||||||
| 177 | 177Er | 177Tm | 177Yb | 177Lu | 177Hf | 177Ta | 177W | 177Re | 177Os | 177Ir | 177Pt | 177Au | 177Hg | 177Tl |
Kernisotone Nuklide des Erbiums
Die zu den Erbium-Kernen isotonen Nuklide befinden sich in der jeweiligen Tabellenzeile; N = Anzahl der Neutronen.
| 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 94 | 95 | 96 | 97 | 98 | 99 | 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 117Mo | ||||||||||||||||||||||||||||||||||
| 118Tc | 119Tc | 120Tc | ||||||||||||||||||||||||||||||||
| 119Ru | 120Ru | 121Ru | 122Ru | 123Ru | 124Ru | |||||||||||||||||||||||||||||
| 120Rh | 121Rh | 122Rh | 123Rh | 124Rh | 125Rh | 126Rh | ||||||||||||||||||||||||||||
| 121Pd | 122Pd | 123Pd | 124Pd | 125Pd | 126Pd | 127Pd | 128Pd | |||||||||||||||||||||||||||
| 122Ag | 123Ag | 124Ag | 125Ag | 126Ag | 127Ag | 128Ag | 129Ag | 130Ag | ||||||||||||||||||||||||||
| 123Cd | 124Cd | 125Cd | 126Cd | 127Cd | 128Cd | 129Cd | 130Cd | 131Cd | 132Cd | 133Cd | ||||||||||||||||||||||||
| 124In | 125In | 126In | 127In | 128In | 129In | 130In | 131In | 132In | 133In | 134In | 135In | |||||||||||||||||||||||
| 125Sn | 126Sn | 127Sn | 128Sn | 129Sn | 130Sn | 131Sn | 132Sn | 133Sn | 134Sn | 135Sn | 136Sn | 137Sn | 138Sn | |||||||||||||||||||||
| 126Sb | 127Sb | 128Sb | 129Sb | 130Sb | 131Sb | 132Sb | 133Sb | 134Sb | 135Sb | 136Sb | 137Sb | 138Sb | 139Sb | 140Sb | ||||||||||||||||||||
| 127Te | 128Te | 129Te | 130Te | 131Te | 132Te | 133Te | 134Te | 135Te | 136Te | 137Te | 138Te | 139Te | 140Te | 141Te | 142Te | 143Te | ||||||||||||||||||
| 128I | 129I | 130I | 131I | 132I | 133I | 134I | 135I | 136I | 137I | 138I | 139I | 140I | 141I | 142I | 143I | 144I | 145I | |||||||||||||||||
| 129Xe | 130Xe | 131Xe | 132Xe | 133Xe | 134Xe | 135Xe | 136Xe | 137Xe | 138Xe | 139Xe | 140Xe | 141Xe | 142Xe | 143Xe | 144Xe | 145Xe | 146Xe | 147Xe | 148Xe | |||||||||||||||
| 130Cs | 131Cs | 132Cs | 133Cs | 134Cs | 135Cs | 136Cs | 137Cs | 138Cs | 139Cs | 140Cs | 141Cs | 142Cs | 143Cs | 144Cs | 145Cs | 146Cs | 147Cs | 148Cs | 149Cs | 150Cs | 151Cs | |||||||||||||
| 131Ba | 132Ba | 133Ba | 134Ba | 135Ba | 136Ba | 137Ba | 138Ba | 139Ba | 140Ba | 141Ba | 142Ba | 143Ba | 144Ba | 145Ba | 146Ba | 147Ba | 148Ba | 149Ba | 150Ba | 151Ba | 152Ba | 153Ba | ||||||||||||
| 132La | 133La | 134La | 135La | 136La | 137La | 138La | 139La | 140La | 141La | 142La | 143La | 144La | 145La | 146La | 147La | 148La | 149La | 150La | 151La | 152La | 153La | 154La | 155La | |||||||||||
| 133Ce | 134Ce | 135Ce | 136Ce | 137Ce | 138Ce | 139Ce | 140Ce | 141Ce | 142Ce | 143Ce | 144Ce | 145Ce | 146Ce | 147Ce | 148Ce | 149Ce | 150Ce | 151Ce | 152Ce | 153Ce | 154Ce | 155Ce | 156Ce | 157Ce | ||||||||||
| 134Pr | 135Pr | 136Pr | 137Pr | 138Pr | 139Pr | 140Pr | 141Pr | 142Pr | 143Pr | 144Pr | 145Pr | 146Pr | 147Pr | 148Pr | 149Pr | 150Pr | 151Pr | 152Pr | 153Pr | 154Pr | 155Pr | 156Pr | 157Pr | 158Pr | 159Pr | |||||||||
| 135Nd | 136Nd | 137Nd | 138Nd | 139Nd | 140Nd | 141Nd | 142Nd | 143Nd | 144Nd | 145Nd | 146Nd | 147Nd | 148Nd | 149Nd | 150Nd | 151Nd | 152Nd | 153Nd | 154Nd | 155Nd | 156Nd | 157Nd | 158Nd | 159Nd | 160Nd | 161Nd | ||||||||
| 136Pm | 137Pm | 138Pm | 139Pm | 140Pm | 141Pm | 142Pm | 143Pm | 144Pm | 145Pm | 146Pm | 147Pm | 148Pm | 149Pm | 150Pm | 151Pm | 152Pm | 153Pm | 154Pm | 155Pm | 156Pm | 157Pm | 158Pm | 159Pm | 160Pm | 161Pm | 162Pm | 163Pm | |||||||
| 137Sm | 138Sm | 139Sm | 140Sm | 141Sm | 142Sm | 143Sm | 144Sm | 145Sm | 146Sm | 147Sm | 148Sm | 149Sm | 150Sm | 151Sm | 152Sm | 153Sm | 154Sm | 155Sm | 156Sm | 157Sm | 158Sm | 159Sm | 160Sm | 161Sm | 162Sm | 163Sm | 164Sm | 165Sm | ||||||
| 138Eu | 139Eu | 140Eu | 141Eu | 142Eu | 143Eu | 144Eu | 145Eu | 146Eu | 147Eu | 148Eu | 149Eu | 150Eu | 151Eu | 152Eu | 153Eu | 154Eu | 155Eu | 156Eu | 157Eu | 158Eu | 159Eu | 160Eu | 161Eu | 162Eu | 163Eu | 164Eu | 165Eu | 166Eu | 167Eu | 168Eu | ||||
| 139Gd | 140Gd | 141Gd | 142Gd | 143Gd | 144Gd | 145Gd | 146Gd | 147Gd | 148Gd | 149Gd | 150Gd | 151Gd | 152Gd | 153Gd | 154Gd | 155Gd | 156Gd | 157Gd | 158Gd | 159Gd | 160Gd | 161Gd | 162Gd | 163Gd | 164Gd | 165Gd | 166Gd | 167Gd | 168Gd | 169Gd | ||||
| 140Tb | 141Tb | 142Tb | 143Tb | 144Tb | 145Tb | 146Tb | 147Tb | 148Tb | 149Tb | 150Tb | 151Tb | 152Tb | 153Tb | 154Tb | 155Tb | 156Tb | 157Tb | 158Tb | 159Tb | 160Tb | 161Tb | 162Tb | 163Tb | 164Tb | 165Tb | 166Tb | 167Tb | 168Tb | 169Tb | 170Tb | 171Tb | |||
| 141Dy | 142Dy | 143Dy | 144Dy | 145Dy | 146Dy | 147Dy | 148Dy | 149Dy | 150Dy | 151Dy | 152Dy | 153Dy | 154Dy | 155Dy | 156Dy | 157Dy | 158Dy | 159Dy | 160Dy | 161Dy | 162Dy | 163Dy | 164Dy | 165Dy | 166Dy | 167Dy | 168Dy | 169Dy | 170Dy | 171Dy | 172Dy | 173Dy | ||
| 142Ho | 143Ho | 144Ho | 145Ho | 146Ho | 147Ho | 148Ho | 149Ho | 150Ho | 151Ho | 152Ho | 153Ho | 154Ho | 155Ho | 156Ho | 157Ho | 158Ho | 159Ho | 160Ho | 161Ho | 162Ho | 163Ho | 164Ho | 165Ho | 166Ho | 167Ho | 168Ho | 169Ho | 170Ho | 171Ho | 172Ho | 173Ho | 174Ho | 175Ho | |
| 143Er | 144Er | 145Er | 146Er | 147Er | 148Er | 149Er | 150Er | 151Er | 152Er | 153Er | 154Er | 155Er | 156Er | 157Er | 158Er | 159Er | 160Er | 161Er | 162Er | 163Er | 164Er | 165Er | 166Er | 167Er | 168Er | 169Er | 170Er | 171Er | 172Er | 173Er | 174Er | 175Er | 176Er | 177Er |
| 144Tm | 145Tm | 146Tm | 147Tm | 148Tm | 149Tm | 150Tm | 151Tm | 152Tm | 153Tm | 154Tm | 155Tm | 156Tm | 157Tm | 158Tm | 159Tm | 160Tm | 161Tm | 162Tm | 163Tm | 164Tm | 165Tm | 166Tm | 167Tm | 168Tm | 169Tm | 170Tm | 171Tm | 172Tm | 173Tm | 174Tm | 175Tm | 176Tm | 177Tm | 178Tm |
| 148Yb | 149Yb | 150Yb | 151Yb | 152Yb | 153Yb | 154Yb | 155Yb | 156Yb | 157Yb | 158Yb | 159Yb | 160Yb | 161Yb | 162Yb | 163Yb | 164Yb | 165Yb | 166Yb | 167Yb | 168Yb | 169Yb | 170Yb | 171Yb | 172Yb | 173Yb | 174Yb | 175Yb | 176Yb | 177Yb | 178Yb | 179Yb | |||
| 150Lu | 151Lu | 152Lu | 153Lu | 154Lu | 155Lu | 156Lu | 157Lu | 158Lu | 159Lu | 160Lu | 161Lu | 162Lu | 163Lu | 164Lu | 165Lu | 166Lu | 167Lu | 168Lu | 169Lu | 170Lu | 171Lu | 172Lu | 173Lu | 174Lu | 175Lu | 176Lu | 177Lu | 178Lu | 179Lu | 180Lu | ||||
| 153Hf | 154Hf | 155Hf | 156Hf | 157Hf | 158Hf | 159Hf | 160Hf | 161Hf | 162Hf | 163Hf | 164Hf | 165Hf | 166Hf | 167Hf | 168Hf | 169Hf | 170Hf | 171Hf | 172Hf | 173Hf | 174Hf | 175Hf | 176Hf | 177Hf | 178Hf | 179Hf | 180Hf | 181Hf | ||||||
| 155Ta | 156Ta | 157Ta | 158Ta | 159Ta | 160Ta | 161Ta | 162Ta | 163Ta | 164Ta | 165Ta | 166Ta | 167Ta | 168Ta | 169Ta | 170Ta | 171Ta | 172Ta | 173Ta | 174Ta | 175Ta | 176Ta | 177Ta | 178Ta | 179Ta | 180Ta | 181Ta | 182Ta | |||||||
| 158W | 159W | 160W | 161W | 162W | 163W | 164W | 165W | 166W | 167W | 168W | 169W | 170W | 171W | 172W | 173W | 174W | 175W | 176W | 177W | 178W | 179W | 180W | 181W | 182W | 183W | |||||||||
| 159Re | 160Re | 161Re | 162Re | 163Re | 164Re | 165Re | 166Re | 167Re | 168Re | 169Re | 170Re | 171Re | 172Re | 173Re | 174Re | 175Re | 176Re | 177Re | 178Re | 179Re | 180Re | 181Re | 182Re | 183Re | 184Re | |||||||||
| 161Os | 162Os | 163Os | 164Os | 165Os | 166Os | 167Os | 168Os | 169Os | 170Os | 171Os | 172Os | 173Os | 174Os | 175Os | 176Os | 177Os | 178Os | 179Os | 180Os | 181Os | 182Os | 183Os | 184Os | 185Os | ||||||||||
| 164Ir | 165Ir | 166Ir | 167Ir | 168Ir | 169Ir | 170Ir | 171Ir | 172Ir | 173Ir | 174Ir | 175Ir | 176Ir | 177Ir | 178Ir | 179Ir | 180Ir | 181Ir | 182Ir | 183Ir | 184Ir | 185Ir | 186Ir | ||||||||||||
| 166Pt | 167Pt | 168Pt | 169Pt | 170Pt | 171Pt | 172Pt | 173Pt | 174Pt | 175Pt | 176Pt | 177Pt | 178Pt | 179Pt | 180Pt | 181Pt | 182Pt | 183Pt | 184Pt | 185Pt | 186Pt | 187Pt | |||||||||||||
| 170Au | 171Au | 172Au | 173Au | 174Au | 175Au | 176Au | 177Au | 178Au | 179Au | 180Au | 181Au | 182Au | 183Au | 184Au | 185Au | 186Au | 187Au | 188Au | ||||||||||||||||
| 171Hg | 172Hg | 173Hg | 174Hg | 175Hg | 176Hg | 177Hg | 178Hg | 179Hg | 180Hg | 181Hg | 182Hg | 183Hg | 184Hg | 185Hg | 186Hg | 187Hg | 188Hg | 189Hg | ||||||||||||||||
| 176Tl | 177Tl | 178Tl | 179Tl | 180Tl | 181Tl | 182Tl | 183Tl | 184Tl | 185Tl | 186Tl | 187Tl | 188Tl | 189Tl | 190Tl | ||||||||||||||||||||
| 178Pb | 179Pb | 180Pb | 181Pb | 182Pb | 183Pb | 184Pb | 185Pb | 186Pb | 187Pb | 188Pb | 189Pb | 190Pb | 191Pb | |||||||||||||||||||||
| 184Bi | 185Bi | 186Bi | 187Bi | 188Bi | 189Bi | 190Bi | 191Bi | 192Bi | ||||||||||||||||||||||||||
| 187Po | 188Po | 189Po | 190Po | 191Po | 192Po | 193Po | ||||||||||||||||||||||||||||
| 191At | 192At | 193At | 194At | |||||||||||||||||||||||||||||||
| 193Rn | 194Rn | 195Rn |
Literatur und Hinweise
Eigenschaften der Erbium-Isotope
[1] - NuDat: National Nuclear Data Center, Brookhaven National Laboratory, based on ENSDF and the Nuclear Wallet Cards.
[2] - G. Audi et. al.: The NUBASE evaluation of nuclear and decay properties. Nuclear Physics, (2003), DOI 10.1016/j.nuclphysa.2003.11.001.
[3] - Live Chart of Nuclides. Nuclear structure and decay data.
Erbium: Kernmagnetische Eigenschaften - 167Er-NMR
[4] - N. J. Stone: Table of nuclear magnetic dipole and electric quadrupole moments. Atomic Data and Nuclear Data Tables, (2005), DOI 10.1016/j.adt.2005.04.001.
[5] - Pekka Pyykkö: Year-2008 nuclear quadrupole moments. Molecular Physics, (2008), DOI 10.1080/00268970802018367.
[6] - Pekka Pyykkö: Year-2017 nuclear quadrupole moments. Molecular Physics, (2018), DOI 10.1080/00268976.2018.1426131.
[7] - N. J. Stone: Table of recommended nuclear magnetic dipole moments. IAEA, (2019).
Weitere Quellen:
[8] - Isotopenhäufigkeiten, Atommassen und Isotopenmassen: Siehe unter dem jeweiligen Stichwort.
[9] - C. Fry, M. Thoennessen:
Discovery of dysprosium, holmium, erbium, thulium and ytterbium isotopes.
In: Atomic Data and Nuclear Data Tables, (2012), DOI 10.1016/j.adt.2012.05.004.
[10] - T. P. D. Swan, P. M. Walker, Zs. Podolyák, M. W. Reed, G. D. Dracoulis, G. J. Lane, T. Kibédi, M. L. Smith:
Discovery of isomers in dysprosium, holmium, and erbium isotopes with N=94 to 97.
In: Physical Review C, (2012), DOI 10.1103/PhysRevC.85.024313.
[11] - Jochen Erler et al.:
Calculated and experimental two-neutron separation energies of even–even erbium isotopes.
In: Nature, (2012), DOI 10.1038/nature11188.
Kategorie: Chemische Elemente
Letzte Änderung am 12.12.2022.
Permalink: https://www.internetchemie.info/chemische-elemente/erbium-isotope.php.
© 1996 - 2026 Internetchemie ChemLin