Unter Titan-Isotope werden alle Atomkerne des chemischen Elements Titan zusammengefasst; diese bestehen allesamt aus einem Atomkern mit 22 Protonen und im ungeladenen Zustand aus 22 Elektronen. Der Unterschied zwischen den einzelnen Titan-Isotopen beruht auf der Anzahl der Neutronen im Kern.
Natürlich auftretende Titan-Isotope
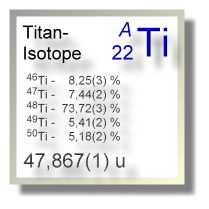
Titan tritt in der Natur in Form seiner stabilen Isotope Ti-46 bis Ti-50 auf; die Atomkerne aller anderen Titanisotope sind künstlich erzeugte Radionuklide.
| Atommasse Ar | Anteil | Halbwertszeit | Spin | |
| Titan Isotopengemisch | 47,867 u | 100 % | ||
| Isotop 46Ti | 45,95262636(10) u | 8,25(3) % | stabil | 0+ |
| Isotop 47Ti | 46,95175749(9) u | 7,44(2) % | stabil | 5/2- |
| Isotop 48Ti | 47,94794068(8) u | 73,72(3) % | stabil | 0+ |
| Isotop 49Ti | 48,94786439(8) u | 5,41(2) % | stabil | 7/2- |
| Isotop 50Ti | 49,94478562(9) u | 5,18(2) % | stabil | 0+ |
Isotopentabelle: Titan
| Isotop Nuklid | Z | A | N | Name | Atommasse [Kernmasse] {Massenüberschuss} | Spin I (h/2π) | μ | A-Nuk |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 38Ti | 22 | 38 | 16 | Titan-38 | 38,01221(32) u [38,0001415 u] {11,37354 MeV} | 0+ | ||
| 39Ti | 22 | 39 | 17 | Titan-39 | 39,002680(220) u [38,9906115 u] {2,4964 MeV} | (3/2+) | 40V | |
| 40Ti | 22 | 40 | 18 | Titan-40 | 39,99050(17) u [39,9784315 u] {-8,84919 MeV} | 0+ | 41V 42Cr | |
| 41Ti | 22 | 41 | 19 | Titan-41 | 40,98315(3) u [40,9710815 u] {-15,69567 MeV} | 3/2+ | 42V | |
| 42Ti | 22 | 42 | 20 | Titan-42 | 41,9730490(3) u [41,9609805 u] {-25,10469 MeV} | 0+ | 43Cr | |
| 43Ti | 22 | 43 | 21 | Titan-43 | 42,968528(6) u [42,9564595 u] {-29,31598 MeV} | 7/2- | 0,85(2) | 43V |
| 43m1Ti | 22 | 43 | 21 | Titan-43m1 | 42,968528(6) u [42,9564595 u] {-29,31598 MeV} | (3/2+) | ||
| 43m2Ti | 22 | 43 | 21 | Titan-43m2 | 42,968528(6) u [42,9564595 u] {-29,31598 MeV} | (19/2-) | +7,22(1) | |
| 44Ti | 22 | 44 | 22 | Titan-44 | 43,9596901(8) u [43,9476216 u] {-37,54843 MeV} | 0+ | 44V | |
| 45Ti | 22 | 45 | 23 | Titan-45 | 44,9581208(9) u [44,9460523 u] {-39,01022 MeV} | 7/2- | 0,095(2) | 45V |
| 45mTi | 22 | 45 | 23 | Titan-45m | 44,9581208(9) u [44,9460523 u] {-39,01022 MeV} | 3/2- | ||
| 46Ti | 22 | 46 | 24 | Titan-46 | 45,95262636(10) u [45,9405578 u] {-44,12826 MeV} | 0+ | 46V 46Sc 46Ca | |
| 47Ti | 22 | 47 | 25 | Titan-47 | 46,95175749(9) u [46,939689 u] {-44,93761 MeV} | 5/2- | -0,78814(11) | 47V 47Sc |
| 48Ti | 22 | 48 | 26 | Titan-48 | 47,94794068(8) u [47,9358722 u] {-48,49294 MeV} | 0+ | 48V 48Sc 48Ca | |
| 49Ti | 22 | 49 | 27 | Titan-49 | 48,94786439(8) u [48,9357959 u] {-48,56401 MeV} | 7/2- | -1,10370(14) | 49V 49Sc |
| 50Ti | 22 | 50 | 28 | Titan-50 | 49,94478562(9) u [49,9327171 u] {-51,43186 MeV} | 0+ | 50V 50Sc 50Cr | |
| 51Ti | 22 | 51 | 29 | Titan-51 | 50,9466096(5) u [50,9345411 u] {-49,73284 MeV} | 3/2- | 51Sc | |
| 52Ti | 22 | 52 | 30 | Titan-52 | 51,946892(8) u [51,9348235 u] {-49,46978 MeV} | 0+ | 52Sc | |
| 53Ti | 22 | 53 | 31 | Titan-53 | 52,94972(11) u [52,9376515 u] {-46,83552 MeV} | (3/2)- | 53Sc | |
| 54Ti | 22 | 54 | 32 | Titan-54 | 53,95102(9) u [53,9389515 u] {-45,62458 MeV} | 0+ | 54Sc | |
| 55Ti | 22 | 55 | 33 | Titan-55 | 54,95527(17) u [54,9432015 u] {-41,66573 MeV} | (1/2)- | 55Sc | |
| 56Ti | 22 | 56 | 34 | Titan-56 | 55,95779(13) u [55,9457215 u] {-39,31836 MeV} | 0+ | 56Sc | |
| 57Ti | 22 | 57 | 35 | Titan-57 | 56,96307(22) u [56,9510015 u] {-34,40007 MeV} | (5/2-) | 57Sc | |
| 58Ti | 22 | 58 | 36 | Titan-58 | 57,96660(21) u [57,9545315 u] {-31,1119 MeV} | 0+ | 58Sc | |
| 59Ti | 22 | 59 | 37 | Titan-59 | 58,97222(32) u [58,9601515 u] {-25,8769 MeV} | (5/2-) | 59Sc | |
| 59mTi | 22 | 59 | 37 | Titan-59m | 58,97222(32) u [58,9601515 u] {-25,8769 MeV} | (1/2-) | ||
| 60Ti | 22 | 60 | 38 | Titan-60 | 59,97603(32) u [59,9639615 u] {-22,32791 MeV} | 0+ | 60Sc | |
| 61Ti | 22 | 61 | 39 | Titan-61 | 60,98245(43) u [60,9703815 u] {-16,34772 MeV} | (1/2-) | ||
| 62Ti | 22 | 62 | 40 | Titan-62 | 61,986900(430) u [61,9748315 u] {-12,20257 MeV} | 0+ | ||
| 63Ti | 22 | 63 | 41 | Titan-63 | 62,99383(54) u [62,9817615 u] {-5,74732 MeV} | 1/2- | ||
| 64Ti | 22 | 64 | 42 | Titan-64 | 63,998410(640) u [63,9863415 u] {-1,48108 MeV} | 0+ | ||
| 65Ti | 22 | 65 | 43 | Titan-65 | 65,005590(750) u [64,9935215 u] {5,20705 MeV} | (1/2-) | ||
| 66Ti | 22 | 66 | 44 | Titan-66 | 66,015 u [66,0029315 u] {13,97241 MeV} | 0+ |
| Isotop | Zerfall (radioaktiver Zerfall) | AE | Mehr | |||
|---|---|---|---|---|---|---|
| Halbwertszeit | Zerfallsart | Anteil | Energie | Info | ||
| 1 | 10 | 11 | 12 | 13 | 14 | 15 |
| Ti-38 | unentdeckt | 2p zu 36Ca | ? | AL | ||
| Ti-39 | 28,5(9) ms | EE β+ zu 39Sc EE p; EE 2p | ? | 16,673(201) MeV | AL | |
| Ti-40 | 52,4(3) ms | EE zu 40Sc EE, p zu 39Ca | 56,99 % 43,01 % | 11,67(16) MeV | AL | |
| Ti-41 | 81,9(5) ms | EE, p zu 40Ca EE zu 41Sc | > 99,9 % < 0,1 % | 12,945(28) MeV | AL | |
| Ti-42 | 208,65(80) ms | EE/β+ zu 42Sc | 100 % | 7,0165(3) MeV | AL | |
| Ti-43 | 509(5) ms | EE/β+ zu 43Sc EE, p 42Ca | 100 % ? | 6,867(7) MeV | AL | |
| Ti-43m1 | 11,9(3) μs | Iso zu 43Ti | 100 % | 313(1) keV | AL | |
| Ti-43m2 | 556(6) ns | Iso zu 43Ti | 100 % | 3066,4(10) keV | AL | |
| Ti-44 | 59,1(3) Jahre | EE zu 44Sc | 100 % | 0,2674(19) MeV | AL | |
| Ti-45 | 184,8(5) Minuten | EE β+ zu 45Sc | 100 % | 2,0621(11) MeV | AL | |
| Ti-45m | 3,0(2) μs | Iso zu 45Ti | 100 % | 36,53(15) keV | AL | |
| Ti-46 | stabil | AL | ||||
| Ti-47 | stabil | AL | ||||
| Ti-48 | stabil | AL | ||||
| Ti-49 | stabil | AL | ||||
| Ti-50 | stabil | AL | ||||
| Ti-51 | 5,76(1) Minuten | β- zu 51V | 100 % | 2,4710(6) MeV | AL | |
| Ti-52 | 1,7(1) Minuten | β- zu 52V | 100 % | 1,974(7) MeV | AL | |
| Ti-53 | 32,7(9) s | β- zu 53V | 100 % | 5,02(10) MeV | AL | |
| Ti-54 | 2,1 s | β- zu 54V | 100 % | 4,27(8) MeV | AL | |
| Ti-55 | 1,3(1) s | β- zu 55V | 100 % | 7,48(16) MeV | AL | |
| Ti-56 | 200(5) ms | β- zu 56V β-, n zu 55V | 100 % | 6,83(19) MeV 1,75(15) MeV | AL | |
| Ti-57 | 98(5) ms | β- zu 57V β-, n zu 56V | 100 % ? | 10,03(22) MeV 3,710(271) MeV | AL | |
| Ti-58 | 58(9) ms | β- zu 58V β-, n zu 57V | 100 % ? | 9,29(22) MeV 5,23(22) MeV | AL | |
| Ti-59 | 28,5(25) ms | β- zu 59V | 100 % | 11,73(33) MeV | AL | |
| Ti-59m | 615(11) ns | Iso zu 59Ti | 100 % | 108,5(5) keV | AL | |
| Ti-60 | 22(2) ms | β- zu 60V β-, n zu 59V β-, 2n zu 58V | 100 % ? ? | 10,91(37) MeV 7,43(34) MeV | AL | |
| Ti-61 | 15(4) ms | β- zu 61V β-, n zu 60V β-, 2n zu 59V | 100 % ? ? | 14,16(98) MeV 8,82(46) MeV | AL | |
| Ti-62 | 9 ms | β-, zu 62V β-, n zu 61V | ? ? | 12,98(50) MeV 9,93(98) MeV | AL | |
| Ti-63 | 360 ns | β-, zu 63V β-, n zu 62V | ? ? | 16,14(64) MeV 11,65(58) MeV | AL | |
| Ti-64 | 5 ms | β-, xn | ? | |||
| Ti-65 | 1 ms | β-, xn | ? | |||
| Ti-66 | ||||||
Erläuterungen zu den einzelnen Spalten:
1 - Symbol mit Nukleonenzahl.
2 - Z = Anzahl der Protonen (Ordnungszahl).
3 - Massenzahl A.
4 - N = Anzahl der Neutronen.
5 - Bezeichnung des Titan-Isotops; gegebenenfalls Trivialnamen.
6 - Relative Atommasse des Titan-Isotops (Isotopenmasse inklusive Elektronen) und in eckigen Klammern die Masse des Atomkerns (Kernmasse, Nuklidmasse ohne Elektronen), jeweils bezogen auf 12C = 12,00000 [2]. Zusätzlich ist der Massenüberschuss (Massenexzess) in MeV angegeben.
7 - Kernspin I, Einheit: h/2π.
8 - Kernmagnetisches Moment μmag.
9 - Ausgangsnuklide: Mögliche, angenommene oder tatsächliche Ausgangs-Nuklide (Mutternuklide, Elternnuklide). Die entsprechenden Zerfalls-Modi sind gegebenenfalls bei den Daten des jeweiligen Ausgangsnuklids zu finden.
10 - Zerfall: Halbwertszeiten des Titan-Isotops mit a = Jahre; ; d = Tage; h = Stunden; min = Minuten; s = Sekunden.
11 - Zerfall: Zerfallsart in die jeweiligen Tochternuklide mit n = Neutronenemission; p = Protonenemission; α = Alpha-Zerfall; ß- = Beta-Minus-Zerfall unter Elektronenemission; EE = Elektroneneinfang; ß+ = Positronenemission; ε = ß+ und/oder EE; Iso = Isomerieübergang; CZ = Cluster-Zerfall; SZ = Spontanzerfall.
12 - Zerfall: Zerfallsanteil in Prozent (%).
13 - Zerfall: Zerfallsenergie; Partikelenergie bezogen auf Zerfallsart.
14 - AE = Anregungsenergie für metastabile Kerne.
15 - Sonstige Informationen und Hinweise: AL = Weitere Niveaus, so genannte Adopted Levels (Verlinkung auf externe Daten [1]).
Sonstige:
()- Eingeklammerte Ziffern: Unsicherheit zur Darstellung der Streubreite des angegebenen Wertes.
~ - Theoretische Werte oder systematische Trends.
- ungelistet-: Nuklide, die in der Literatur bereits erwänhnt wurden, aber aus irgendwelchen Gründen in den aktuellen Nuklidtabellen nicht mehr zu finden sind, weil sich deren Entdeckung z. B. nicht bestätigt hat.
NMR-aktive Titan-Nuklide
| Nuklid Anteil Spin I | Kernmagnetisches Moment μ/μN | Gyromagnetisches Verhältnis 107 rad T-1 s-1 | Quadrupol- Moment Q [barn] | Resonanz- Frequenz v0 bei 1 T | Relative Empfindlichkeit H0 = const. v0 = const. * |
|---|---|---|---|---|---|
| 47Ti 7,44(2) % 5/2- | -0,78814(11) | -1,5105 | +0,302(10) | 2,4041 | 0,00210 0,6588 |
| 49Ti 5,41(2) % 7/2- | -1,10370(14) | - 1,51095 | + 0,247(11) | 2,4048 | 0,00378 1,1861 |
*) bezogen auf 1H = 1,000
Strahlenschutz
Für den Umgang mit den Titan-Radionukliden gelten gemäß Strahlenschutzverordnung (StrlSchV 2018) unter anderem folgende Werte (Spalten 1 bis 7):
| Nuklid | Freigrenzen | HRQ-Schwelle | OFK | Tochternuklide | Halbwertszeit | |
|---|---|---|---|---|---|---|
| Ti-44+ | 105 Bq | 0,1 Bq/g | 0,03 TBq | Sc-44 | 60,0 Jahre | |
| Ti-45 | 106 Bq | 10 Bq/g | 3,1 Stunden | |||
(HRQ = Hochradioaktive Quellen; OFK = Oberflächenkontamination)
Kernisobare Nuklide des Titans
Zu den Titan-Nukliden isobare Atomkerne befinden sich in der jeweiligen Tabellenzeile; Z = Ordnungszahl; A = Nukleonenzahl (Massenzahl).
| Z: | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Mg | Al | Si | P | S | Cl | Ar | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As |
| 38 | 38Mg | 38Al | 38Si | 38P | 38S | 38Cl | 38Ar | 38K | 38Ca | 38Sc | 38Ti | |||||||||||
| 39 | 39Mg | 39Al | 39Si | 39P | 39S | 39Cl | 39Ar | 39K | 39Ca | 39Sc | 39Ti | |||||||||||
| 40 | 40Mg | 40Al | 40Si | 40P | 40S | 40Cl | 40Ar | 40K | 40Ca | 40Sc | 40Ti | 40V | ||||||||||
| 41 | 41Al | 41Si | 41P | 41S | 41Cl | 41Ar | 41K | 41Ca | 41Sc | 41Ti | 41V | 41Cr | ||||||||||
| 42 | 42Al | 42Si | 42P | 42S | 42Cl | 42Ar | 42K | 42Ca | 42Sc | 42Ti | 42V | 42Cr | 42Mn | |||||||||
| 43 | 43Al | 43Si | 43P | 43S | 43Cl | 43Ar | 43K | 43Ca | 43Sc | 43Ti | 43V | 43Cr | 43Mn | |||||||||
| 44 | 44Si | 44P | 44S | 44Cl | 44Ar | 44K | 44Ca | 44Sc | 44Ti | 44V | 44Cr | 44Mn | ||||||||||
| 45 | 45P | 45S | 45Cl | 45Ar | 45K | 45Ca | 45Sc | 45Ti | 45V | 45Cr | 45Mn | 45Fe | ||||||||||
| 46 | 46P | 46S | 46Cl | 46Ar | 46K | 46Ca | 46Sc | 46Ti | 46V | 46Cr | 46Mn | 46Fe | 46Co | |||||||||
| 47 | 47S | 47Cl | 47Ar | 47K | 47Ca | 47Sc | 47Ti | 47V | 47Cr | 47Mn | 47Fe | 47Co | ||||||||||
| 48 | 48S | 48Cl | 48Ar | 48K | 48Ca | 48Sc | 48Ti | 48V | 48Cr | 48Mn | 48Fe | 48Co | 48Ni | |||||||||
| 49 | 49S | 49Cl | 49Ar | 49K | 49Ca | 49Sc | 49Ti | 49V | 49Cr | 49Mn | 49Fe | 49Co | 49Ni | |||||||||
| 50 | 50Cl | 50Ar | 50K | 50Ca | 50Sc | 50Ti | 50V | 50Cr | 50Mn | 50Fe | 50Co | 50Ni | ||||||||||
| 51 | 51Cl | 51Ar | 51K | 51Ca | 51Sc | 51Ti | 51V | 51Cr | 51Mn | 51Fe | 51Co | 51Ni | ||||||||||
| 52 | 52Ar | 52K | 52Ca | 52Sc | 52Ti | 52V | 52Cr | 52Mn | 52Fe | 52Co | 52Ni | |||||||||||
| 53 | 53Ar | 53K | 53Ca | 53Sc | 53Ti | 53V | 53Cr | 53Mn | 53Fe | 53Co | 53Ni | 53Cu | ||||||||||
| 54 | 54K | 54Ca | 54Sc | 54Ti | 54V | 54Cr | 54Mn | 54Fe | 54Co | 54Ni | 54Cu | 54Zn | ||||||||||
| 55 | 55K | 55Ca | 55Sc | 55Ti | 55V | 55Cr | 55Mn | 55Fe | 55Co | 55Ni | 55Cu | 55Zn | ||||||||||
| 56 | 56K | 56Ca | 56Sc | 56Ti | 56V | 56Cr | 56Mn | 56Fe | 56Co | 56Ni | 56Cu | 56Zn | ||||||||||
| 57 | 57Ca | 57Sc | 57Ti | 57V | 57Cr | 57Mn | 57Fe | 57Co | 57Ni | 57Cu | 57Zn | |||||||||||
| 58 | 58Ca | 58Sc | 58Ti | 58V | 58Cr | 58Mn | 58Fe | 58Co | 58Ni | 58Cu | 58Zn | |||||||||||
| 59 | 59Sc | 59Ti | 59V | 59Cr | 59Mn | 59Fe | 59Co | 59Ni | 59Cu | 59Zn | 59Ga | |||||||||||
| 60 | 60Sc | 60Ti | 60V | 60Cr | 60Mn | 60Fe | 60Co | 60Ni | 60Cu | 60Zn | 60Ga | 60Ge | ||||||||||
| 61 | 61Sc | 61Ti | 61V | 61Cr | 61Mn | 61Fe | 61Co | 61Ni | 61Cu | 61Zn | 61Ga | 61Ge | ||||||||||
| 62 | 62Ti | 62V | 62Cr | 62Mn | 62Fe | 62Co | 62Ni | 62Cu | 62Zn | 62Ga | 62Ge | 62As | ||||||||||
| 63 | 63Ti | 63V | 63Cr | 63Mn | 63Fe | 63Co | 63Ni | 63Cu | 63Zn | 63Ga | 63Ge | 63As |
Kernisotone Nuklide des Titans
Die zu den Titan-Kernen isotonen Nuklide befinden sich in der jeweiligen Tabellenzeile; N = Anzahl der Neutronen.
| 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21B | |||||||||||||||||||||||||
| 22C | |||||||||||||||||||||||||
| 23N | 24N | 25N | |||||||||||||||||||||||
| 24O | 25O | 26O | |||||||||||||||||||||||
| 25F | 26F | 27F | 28F | 29F | 30F | 31F | |||||||||||||||||||
| 26Ne | 27Ne | 28Ne | 29Ne | 30Ne | 31Ne | 32Ne | 33Ne | 34Ne | |||||||||||||||||
| 27Na | 28Na | 29Na | 30Na | 31Na | 32Na | 33Na | 34Na | 35Na | 36Na | 37Na | 38Na | 39Na | |||||||||||||
| 28Mg | 29Mg | 30Mg | 31Mg | 32Mg | 33Mg | 34Mg | 35Mg | 36Mg | 37Mg | 38Mg | 39Mg | 40Mg | |||||||||||||
| 29Al | 30Al | 31Al | 32Al | 33Al | 34Al | 35Al | 36Al | 37Al | 38Al | 39Al | 40Al | 41Al | 42Al | 43Al | |||||||||||
| 30Si | 31Si | 32Si | 33Si | 34Si | 35Si | 36Si | 37Si | 38Si | 39Si | 40Si | 41Si | 42Si | 43Si | 44Si | |||||||||||
| 31P | 32P | 33P | 34P | 35P | 36P | 37P | 38P | 39P | 40P | 41P | 42P | 43P | 44P | 45P | 46P | ||||||||||
| 32S | 33S | 34S | 35S | 36S | 37S | 38S | 39S | 40S | 41S | 42S | 43S | 44S | 45S | 46S | 47S | 48S | 49S | ||||||||
| 33Cl | 34Cl | 35Cl | 36Cl | 37Cl | 38Cl | 39Cl | 40Cl | 41Cl | 42Cl | 43Cl | 44Cl | 45Cl | 46Cl | 47Cl | 48Cl | 49Cl | 50Cl | 51Cl | |||||||
| 34Ar | 35Ar | 36Ar | 37Ar | 38Ar | 39Ar | 40Ar | 41Ar | 42Ar | 43Ar | 44Ar | 45Ar | 46Ar | 47Ar | 48Ar | 49Ar | 50Ar | 51Ar | 52Ar | 53Ar | ||||||
| 35K | 36K | 37K | 38K | 39K | 40K | 41K | 42K | 43K | 44K | 45K | 46K | 47K | 48K | 49K | 50K | 51K | 52K | 53K | 54K | 55K | 56K | ||||
| 36Ca | 37Ca | 38Ca | 39Ca | 40Ca | 41Ca | 42Ca | 43Ca | 44Ca | 45Ca | 46Ca | 47Ca | 48Ca | 49Ca | 50Ca | 51Ca | 52Ca | 53Ca | 54Ca | 55Ca | 56Ca | 57Ca | 58Ca | |||
| 37Sc | 38Sc | 39Sc | 40Sc | 41Sc | 42Sc | 43Sc | 44Sc | 45Sc | 46Sc | 47Sc | 48Sc | 49Sc | 50Sc | 51Sc | 52Sc | 53Sc | 54Sc | 55Sc | 56Sc | 57Sc | 58Sc | 59Sc | 60Sc | 61Sc | |
| 38Ti | 39Ti | 40Ti | 41Ti | 42Ti | 43Ti | 44Ti | 45Ti | 46Ti | 47Ti | 48Ti | 49Ti | 50Ti | 51Ti | 52Ti | 53Ti | 54Ti | 55Ti | 56Ti | 57Ti | 58Ti | 59Ti | 60Ti | 61Ti | 62Ti | 63Ti |
| 40V | 41V | 42V | 43V | 44V | 45V | 46V | 47V | 48V | 49V | 50V | 51V | 52V | 53V | 54V | 55V | 56V | 57V | 58V | 59V | 60V | 61V | 62V | 63V | 64V | |
| 41Cr | 42Cr | 43Cr | 44Cr | 45Cr | 46Cr | 47Cr | 48Cr | 49Cr | 50Cr | 51Cr | 52Cr | 53Cr | 54Cr | 55Cr | 56Cr | 57Cr | 58Cr | 59Cr | 60Cr | 61Cr | 62Cr | 63Cr | 64Cr | 65Cr | |
| 42Mn | 43Mn | 44Mn | 45Mn | 46Mn | 47Mn | 48Mn | 49Mn | 50Mn | 51Mn | 52Mn | 53Mn | 54Mn | 55Mn | 56Mn | 57Mn | 58Mn | 59Mn | 60Mn | 61Mn | 62Mn | 63Mn | 64Mn | 65Mn | 66Mn | |
| 45Fe | 46Fe | 47Fe | 48Fe | 49Fe | 50Fe | 51Fe | 52Fe | 53Fe | 54Fe | 55Fe | 56Fe | 57Fe | 58Fe | 59Fe | 60Fe | 61Fe | 62Fe | 63Fe | 64Fe | 65Fe | 66Fe | 67Fe | |||
| 46Co | 47Co | 48Co | 49Co | 50Co | 51Co | 52Co | 53Co | 54Co | 55Co | 56Co | 57Co | 58Co | 59Co | 60Co | 61Co | 62Co | 63Co | 64Co | 65Co | 66Co | 67Co | 68Co | |||
| 48Ni | 49Ni | 50Ni | 51Ni | 52Ni | 53Ni | 54Ni | 55Ni | 56Ni | 57Ni | 58Ni | 59Ni | 60Ni | 61Ni | 62Ni | 63Ni | 64Ni | 65Ni | 66Ni | 67Ni | 68Ni | 69Ni | ||||
| 53Cu | 54Cu | 55Cu | 56Cu | 57Cu | 58Cu | 59Cu | 60Cu | 61Cu | 62Cu | 63Cu | 64Cu | 65Cu | 66Cu | 67Cu | 68Cu | 69Cu | 70Cu | ||||||||
| 54Zn | 55Zn | 56Zn | 57Zn | 58Zn | 59Zn | 60Zn | 61Zn | 62Zn | 63Zn | 64Zn | 65Zn | 66Zn | 67Zn | 68Zn | 69Zn | 70Zn | 71Zn | ||||||||
| 59Ga | 60Ga | 61Ga | 62Ga | 63Ga | 64Ga | 65Ga | 66Ga | 67Ga | 68Ga | 69Ga | 70Ga | 71Ga | 72Ga | ||||||||||||
| 60Ge | 61Ge | 62Ge | 63Ge | 64Ge | 65Ge | 66Ge | 67Ge | 68Ge | 69Ge | 70Ge | 71Ge | 72Ge | 73Ge | ||||||||||||
| 62As | 63As | 64As | 65As | 66As | 67As | 68As | 69As | 70As | 71As | 72As | 73As | 74As | |||||||||||||
| 65Se | 66Se | 67Se | 68Se | 69Se | 70Se | 71Se | 72Se | 73Se | 74Se | 75Se | |||||||||||||||
| 66Br | 67Br | 68Br | 69Br | 70Br | 71Br | 72Br | 73Br | 74Br | 75Br | 76Br | |||||||||||||||
| 69Kr | 70Kr | 71Kr | 72Kr | 73Kr | 74Kr | 75Kr | 76Kr | 77Kr | |||||||||||||||||
| 71Rb | 72Rb | 73Rb | 74Rb | 75Rb | 76Rb | 77Rb | 78Rb | ||||||||||||||||||
| 73Sr | 74Sr | 75Sr | 76Sr | 77Sr | 78Sr | 79Sr | |||||||||||||||||||
| 76Y | 77Y | 78Y | 79Y | 80Y | |||||||||||||||||||||
| 78Zr | 79Zr | 80Zr | 81Zr | ||||||||||||||||||||||
| 81Nb | 82Nb | ||||||||||||||||||||||||
| 83Mo |
Literatur und Hinweise
Eigenschaften der Titan-Isotope
[1] - NuDat: National Nuclear Data Center, Brookhaven National Laboratory, based on ENSDF and the Nuclear Wallet Cards.
[2] - G. Audi et. al.: The NUBASE evaluation of nuclear and decay properties. Nuclear Physics, (2003), DOI 10.1016/j.nuclphysa.2003.11.001.
[3] - Live Chart of Nuclides. Nuclear structure and decay data.
Titan: Kernmagnetische Eigenschaften - 47Ti-NMR, 49Ti-NMR
[4] - N. J. Stone: Table of nuclear magnetic dipole and electric quadrupole moments. Atomic Data and Nuclear Data Tables, (2005), DOI 10.1016/j.adt.2005.04.001.
[5] - Pekka Pyykkö: Year-2008 nuclear quadrupole moments. Molecular Physics, (2008), DOI 10.1080/00268970802018367.
[6] - Pekka Pyykkö: Year-2017 nuclear quadrupole moments. Molecular Physics, (2018), DOI 10.1080/00268976.2018.1426131.
[7] - N. J. Stone: Table of recommended nuclear magnetic dipole moments. IAEA, (2019).
Weitere Quellen:
[8] - Isotopenhäufigkeiten, Atommassen und Isotopenmassen: Siehe unter dem jeweiligen Stichwort.
[9] - D. Meierfrankenfeld, A. Bury, M. Thoennessen:
Discovery of scandium, titanium, mercury, and einsteinium isotopes.
In: Atomic Data and Nuclear Data Tables, (2011), DOI 10.1016/j.adt.2010.11.001.
Kategorie: Chemische Elemente
Letzte Änderung am 12.12.2022.
Permalink: https://www.internetchemie.info/chemische-elemente/titan-isotope.php.
© 1996 - 2026 Internetchemie ChemLin