Unter Germanium-Isotope werden alle Atomkerne des chemischen Elements Germanium zusammengefasst; diese bestehen aus einem Atomkern mit 32 Protonen und im ungeladenen Zustand aus 32 Elektronen. Der Unterschied zwischen den einzelnen Germanium-Isotopen liegt in der Anzahl der Neutronen im Kern begründet.
Natürlich auftretende Germanium-Isotope
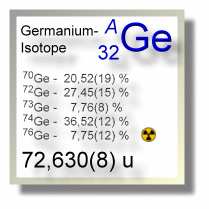
Natürliche Vorkommen des Germaniums setzten sich aus den 5 Isotopen Ge-70, Ge-72, Ge-73, Ge-74 und Ge-76 zusammen, von denen das Germanium-76 mit einer Halbwertszeit von 130 Billionen Jahren zerfällt und daher leicht radioaktiv ist; alle anderen natürlichen Germaniumnuklide gelten als stabil.
| Atommasse Ar | Anteil | Halbwertszeit | Spin | |
| Germanium Isotopengemisch | 72,630 u | 100 % | ||
| Isotop 70Ge | 69,924249(6) u | 20,52(19) % | stabil | 0+ |
| Isotop 72Ge | 71,9220758(5) u | 27,45(15) % | stabil | 0+ |
| Isotop 73Ge | 72,92345895(6) u | 7,76(8) % | stabil | 9/2+ |
| Isotop 74Ge | 73,92117776(9) u | 36,52(12) % | stabil | 0+ |
| Isotop 76Ge | 75,9214027(2) u | 7,75(12) % | 1,926(94) × 1021 Jahre | 0+ |
Darüber hinaus konnten 27 weitere Germanium-Radioisotope charakterisiert werden.
Isotopentabelle: Germanium
| Isotop Nuklid | Z | A | N | Name | Atommasse [Kernmasse] {Massenüberschuss} | Spin I (h/2π) | μ | A-Nuk |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 59Ge | 32 | 59 | 27 | Germanium-59 | 58,98296(43) u [58,965406 u] {-15,87266 MeV} | 7/2- | ||
| 60Ge | 32 | 60 | 28 | Germanium-60 | 59,97092(32) u [59,953366 u] {-27,08785 MeV} | 0+ | ||
| 61Ge | 32 | 61 | 29 | Germanium-61 | 60,96419(32) u [60,946636 u] {-33,3568 MeV} | (3/2-) | ||
| 62Ge | 32 | 62 | 30 | Germanium-62 | 61,95519(15) u [61,937636 u] {-41,74025 MeV} | 0+ | ||
| 63Ge | 32 | 63 | 31 | Germanium-63 | 62,94963(4) u [62,932076 u] {-46,91935 MeV} | 3/2- | ||
| 64Ge | 32 | 64 | 32 | Germanium-64 | 63,94169(4) u [63,924136 u] {-54,31542 MeV} | 0+ | ||
| 65Ge | 32 | 65 | 33 | Germanium-65 | 64,9393681(23) u [64,9218141 u] {-56,47825 MeV} | 3/2- | ||
| 66Ge | 32 | 66 | 34 | Germanium-66 | 65,9338621(26) u [65,9163081 u] {-61,60706 MeV} | 0+ | 66As | |
| 67Ge | 32 | 67 | 35 | Germanium-67 | 66,932734(5) u [66,91518 u] {-62,65788 MeV} | 1/2- | 67As | |
| 67m1Ge | 32 | 67 | 35 | Germanium-67m1 | 66,932734(5) u [66,91518 u] {-62,65788 MeV} | 5/2- | ||
| 67m2Ge | 32 | 67 | 35 | Germanium-67m2 | 66,932734(5) u [66,91518 u] {-62,65788 MeV} | 9/2+ | -0,849(18) | |
| 68Ge | 32 | 68 | 36 | Germanium-68 | 67,9280953(20) u [67,9105413 u] {-66,9788 MeV} | 0+ | 68As 69Se | |
| 69Ge | 32 | 69 | 37 | Germanium-69 | 68,9279645(14) u [68,9104105 u] {-67,10064 MeV} | 5/2- | 0,735(7) | 69As |
| 69m1Ge | 32 | 69 | 37 | Germanium-69m1 | 68,9279645(14) u [68,9104105 u] {-67,10064 MeV} | 1/2- | ||
| 69m2Ge | 32 | 69 | 37 | Germanium-69m2 | 68,9279645(14) u [68,9104105 u] {-67,10064 MeV} | 9/2+ | -1,001(3) | |
| 70Ge | 32 | 70 | 38 | Germanium-70 | 69,924249(6) u [69,906695 u] {-70,5616 MeV} | 0+ | 70As 70Ga | |
| 71Ge | 32 | 71 | 39 | Germanium-71 | 70,9249521(9) u [70,9073981 u] {-69,90667 MeV} | 1/2- | + 0,546(5) | 71As |
| 71mGe | 32 | 71 | 39 | Germanium-71m | 70,9249521(9) u [70,9073981 u] {-69,90667 MeV} | 9/2+ | - 1,039(2) | |
| 72Ge | 32 | 72 | 40 | Germanium-72 | 71,9220758(5) u [71,9045218 u] {-72,58592 MeV} | 0+ | 72As 72Ga | |
| 72mGe | 32 | 72 | 40 | Germanium-72m | 71,9220758(5) u [71,9045218 u] {-72,58592 MeV} | 0+ | ||
| 73Ge | 32 | 73 | 41 | Germanium-73 | 72,92345895(6) u [72,905905 u] {-71,29753 MeV} | 9/2+ | -0,87824(5) | 73As 73Ga |
| 73m1Ge | 32 | 73 | 41 | Germanium-73m1 | 72,9234590(4) u [72,905905 u] {-71,29748 MeV} | 5/2+ | -1,08(3) | |
| 73m2Ge | 32 | 73 | 41 | Germanium-73m2 | 72,9234590(4) u [72,905905 u] {-71,29748 MeV} | 1/2- | ||
| 74Ge | 32 | 74 | 42 | Germanium-74 | 73,92117776(9) u [73,9036238 u] {-73,42244 MeV} | 0+ | 74As 74Ga | |
| 75Ge | 32 | 75 | 43 | Germanium-75 | 74,92285837(5) u [74,9053044 u] {-71,85697 MeV} | 1/2- | + 0,509(5) | 75Ga |
| 75m1Ge | 32 | 75 | 43 | Germanium-75m1 | 74,92285837(5) u [74,9053044 u] {-71,85697 MeV} | 7/2+ | ||
| 75m2Ge | 32 | 75 | 43 | Germanium-75m2 | 74,92285837(5) u [74,9053044 u] {-71,85697 MeV} | 5/2+ | ||
| 76Ge | 32 | 76 | 44 | Germanium-76 | 75,9214027(2) u [75,9038487 u] {-73,21291 MeV} | 0+ | 76Ga | |
| 77Ge | 32 | 77 | 45 | Germanium-77 | 76,92354984(6) u [76,9059959 u] {-71,21287 MeV} | 7/2+ | 77Ga | |
| 77mGe | 32 | 77 | 45 | Germanium-77m | 76,92354984(6) u [76,9059959 u] {-71,21287 MeV} | 1/2- | ||
| 78Ge | 32 | 78 | 46 | Germanium-78 | 77,922853(4) u [77,905299 u] {-71,86197 MeV} | 0+ | 78Ga 79Ga | |
| 79Ge | 32 | 79 | 47 | Germanium-79 | 78,92536(4) u [78,907806 u] {-69,52671 MeV} | (1/2)- | 79Ga 80Ga | |
| 79mGe | 32 | 79 | 47 | Germanium-79m | 78,92536(4) u [78,907806 u] {-69,52671 MeV} | (7/2+) | ||
| 80Ge | 32 | 80 | 48 | Germanium-80 | 79,9253508(22) u [79,9077968 u] {-69,53528 MeV} | 0+ | 80Ga 81Ga | |
| 81Ge | 32 | 81 | 49 | Germanium-81 | 80,9288329(22) u [80,9112789 u] {-66,29173 MeV} | (9/2+) | 81Ga 82Ga | |
| 81mGe | 32 | 81 | 49 | Germanium-81m | 80,9288329(22) u [80,9112789 u] {-66,29173 MeV} | (1/2+) | ||
| 82Ge | 32 | 82 | 50 | Germanium-82 | 81,9297740(24) u [81,91222 u] {-65,4151 MeV} | 0+ | 82Ga 83Ga | |
| 83Ge | 32 | 83 | 51 | Germanium-83 | 82,9345391(26) u [82,9169851 u] {-60,97644 MeV} | (5/2)+ | ||
| 84Ge | 32 | 84 | 52 | Germanium-84 | 83,937575(3) u [83,920021 u] {-58,14851 MeV} | 0+ | ||
| 85Ge | 32 | 85 | 53 | Germanium-85 | 84,942970(4) u [84,925416 u] {-53,1231 MeV} | (3/2+,5/2+) | ||
| 86Ge | 32 | 86 | 54 | Germanium-86 | 85,94697(47) u [85,929416 u] {-49,39713 MeV} | 0+ | ||
| 87Ge | 32 | 87 | 55 | Germanium-87 | 86,95268(32) u [86,935126 u] {-44,0783 MeV} | (5/2+) | ||
| 88Ge | 32 | 88 | 56 | Germanium-88 | 87,95691(43) u [87,939356 u] {-40,13808 MeV} | 0+ | ||
| 89Ge | 32 | 89 | 57 | Germanium-89 | 88,96379(43) u [88,946236 u] {-33,7294 MeV} | 3/2+ | ||
| 90Ge | 32 | 90 | 58 | Germanium-90 | 89,96863(54) u [89,951076 u] {-29,22097 MeV} | 0+ |
| Isotop | Zerfall (radioaktiver Zerfall) | AE | Mehr | |||
|---|---|---|---|---|---|---|
| Halbwertszeit | Zerfallsart | Anteil | Energie | Info | ||
| 1 | 10 | 11 | 12 | 13 | 14 | 15 |
| Ge-59 | 13,3(17) ms | 2 p zu 57Zn EE/β+ zu 58Ga | < 0,2 % ? | 17,89(43) MeV | ||
| Ge-60 | 110 ns | EE/β+ zu 60Ga EE, p 59Zn | ? ? | 12,50(36) MeV | AL | |
| Ge-61 | 44(6) ms | EE/β+ zu 61Ga EE, p zu 60Zn | < 38 % > 62 % | 13,77(30) MeV | AL | |
| Ge-62 | 129(35) ms | EE/β+ zu 62Ga EE, p zu 61Zn | ? | 10,25(14) MeV | AL | |
| Ge-63 | 150(9) ms | EE/β+ zu 63Ga | 100 % | 9,63(40) MeV | AL | |
| Ge-64 | 63,7(25) s | EE/β+ zu 64Ga | 100 % | 4,517(4) MeV | AL | |
| Ge-65 | 30,9(5) s | EE/β+ zu 65Ga EE, p zu 65Zn | 99,9 % 0,011(3) % | 6,1793(23) MeV | AL | |
| Ge-66 | 2,26(5) Stunden | EE/β+ zu 66Ga | 100 % | 2,1166(26) MeV | AL | |
| Ge-67 | 18,9(3) Minuten | EE/β+ zu 67Ga | 100 % | 4,221(5) MeV | AL | |
| Ge-67m1 | 13,7(9) μs | 18,20(5) keV | ||||
| Ge-67m2 | 146(4) ns | 751,70(6) keV | ||||
| Ge-68 | 271,05(8) Tage | EE zu 68Ga | 100 % | 0,1073(24) MeV | AL | |
| Ge-69 | 39,05(10) Stunden | EE/β+ zu 69Ga | 100 % | 2,2271(18) MeV | AL | |
| Ge-69m1 | 5,1(2) μs | Iso zu 69Ge | 100 % | 86,76(2) keV | ||
| Ge-69m2 | 2,81(5) μs | Iso zu 69Ge | 100 % | 397,94(2) keV | ||
| Ge-70 | stabil | AL | ||||
| Ge-71 | 11,468(8) d | EE zu 71Ga | 100 % | 0,2325(12) MeV | AL | |
| Ge-71m | 20,22(12) ms | Iso zu 71Ge | 100 % | 198,371(12) keV | AL | |
| Ge-72 | stabil | AL | ||||
| Ge-72m | 444,2(8) ns | 691,43(4) keV | ||||
| Ge-73 | stabil | AL | ||||
| Ge-73m1 | 0,499(11) s | 13,2845(15) keV | AL | |||
| Ge-73m2 | 499(11) ms | Iso zu 73Ge | 100 % | 66,725(9) keV | ||
| Ge-74 | stabil | AL | ||||
| Ge-75 | 82,78(4) Minuten | β- zu 75As | 100 % | 11,772(9) MeV | AL | |
| Ge-75m1 | 47,7(5) s | β- zu 75As Iso zu 75Ge | 0,030(6) % 99,970(6) % | 139,69(3) KeV | AL | |
| Ge-75m2 | 216(5) ns | 192,19(6) keV | ||||
| Ge-76 | 1,926(94) × 1021 Jahre | β- β- zu 76Se | AL | |||
| Ge-77 | 11,211(3) Stunden | β- zu 77As | 100 % | 2,7035(17) MeV | AL | |
| Ge-77m | 53,7(6) s | β- zu 77As Iso zu 77Ge | 81(2) % 19(2) % | 159,71(6) | AL | |
| Ge-78 | 88,0(10) Minuten | β- zu 78As | 100 % | 0,955(10) MeV | AL | |
| Ge-79 | 18,98(3) s | β- zu 79As | 100 % | 4,11(4) MeV | AL | |
| Ge-79m | 39,0(10) s | β- zu 79As Iso zu 79Ge | 96(1) % 4(1) % | 185,95(4) keV | AL | |
| Ge-80 | 29,5(4) s | β- zu 80As | 100 % | 2,679(4) MeV | AL | |
| Ge-81 | 7,6(6) s | β- zu 81As | 100 % | 6,242(3) MeV | AL | |
| Ge-81m | 7,6(6) s | β- zu 81As | 100 % | 679,14(4) keV | AL | |
| Ge-82 | 4,0(7) s | β- zu 82As | 100 % | 4,690(4) MeV | AL | |
| Ge-83 | 1,85(6) s | β- zu 83As β-, n zu 82As | 100 % ? | 8,693(4) MeV 1,058(4) MeV | AL | |
| Ge-84 | 954(14) ms | β- zu 84As β-, n zu 83As | 89,8 % 10,2(9) % | 7,705(4) MeV 3,450(4) MeV | AL | |
| Ge-85 | 503(18) ms | β- zu 85As β-, n zu 84As β-, 2n zu 83As | 83,5 % 16,5(23) % ? | 10,066(5) MeV 4,659(5) MeV | AL | |
| Ge-86 | 226(21) ms | β- zu 86As β-, n zu 85As | 55 % 45(15) % | 9,56(44) MeV 5,72(44) MeV | AL | |
| Ge-87 | 0,14 s | β- zu 87As β-, n zu 86As | 100 % ? | 11,54(30) MeV 6,81(30) MeV | AL | |
| Ge-88 | 300 ns | β- zu 88As β-, n zu 87As | ? ? | 10,58(44) MeV 7,41(40) MeV | AL | |
| Ge-89 | 300 ns | β- zu 89As β-, n zu 88As β-, 2n zu 87As | ? ? ? | 13,07(50) MeV 8,92(44) MeV | AL | |
| Ge-90 | 635 ns | β- zu 90As β-, n zu 89As β-, 2n zu 88As | ? ? ? | 12,11(64) MeV 9,51(58) MeV | ||
Erläuterungen zu den einzelnen Spalten:
1 - Symbol mit Nukleonenzahl.
2 - Z = Anzahl der Protonen (Ordnungszahl).
3 - Massenzahl A.
4 - N = Anzahl der Neutronen.
5 - Bezeichnung des Germanium-Isotops; gegebenenfalls Trivialnamen.
6 - Relative Atommasse des Germanium-Isotops (Isotopenmasse inklusive Elektronen) und in eckigen Klammern die Masse des Atomkerns (Kernmasse, Nuklidmasse ohne Elektronen), jeweils bezogen auf 12C = 12,00000 [2]. Zusätzlich ist der Massenüberschuss (Massenexzess) in MeV angegeben.
7 - Kernspin I, Einheit: h/2π.
8 - Kernmagnetisches Moment μmag.
9 - Ausgangsnuklide: Mögliche, angenommene oder tatsächliche Ausgangs-Nuklide (Mutternuklide, Elternnuklide). Die entsprechenden Zerfalls-Modi sind gegebenenfalls bei den Daten des jeweiligen Ausgangsnuklids zu finden.
10 - Zerfall: Halbwertszeiten des Germanium-Isotops mit a = Jahre; ; d = Tage; h = Stunden; min = Minuten; s = Sekunden.
11 - Zerfall: Zerfallsart in die jeweiligen Tochternuklide mit n = Neutronenemission; p = Protonenemission; α = Alpha-Zerfall; ß- = Beta-Minus-Zerfall unter Elektronenemission; EE = Elektroneneinfang; ß+ = Positronenemission; ε = ß+ und/oder EE; Iso = Isomerieübergang; CZ = Cluster-Zerfall; SZ = Spontanzerfall.
12 - Zerfall: Zerfallsanteil in Prozent (%).
13 - Zerfall: Zerfallsenergie; Partikelenergie bezogen auf Zerfallsart.
14 - AE = Anregungsenergie für metastabile Kerne.
15 - Sonstige Informationen und Hinweise: AL = Weitere Niveaus, so genannte Adopted Levels (Verlinkung auf externe Daten [1]).
Sonstige:
()- Eingeklammerte Ziffern: Unsicherheit zur Darstellung der Streubreite des angegebenen Wertes.
~ - Theoretische Werte oder systematische Trends.
- ungelistet-: Nuklide, die in der Literatur bereits erwänhnt wurden, aber aus irgendwelchen Gründen in den aktuellen Nuklidtabellen nicht mehr zu finden sind, weil sich deren Entdeckung z. B. nicht bestätigt hat.
NMR-aktive Germanium-Nuklide
| Nuklid Anteil Spin I | Kernmagnetisches Moment μ/μN | Gyromagnetisches Verhältnis 107 rad T-1 s-1 | Quadrupol- Moment Q [barn] | Resonanz- Frequenz v0 bei 1 T | Relative Empfindlichkeit H0 = const. v0 = const. * |
|---|---|---|---|---|---|
| 73Ge 7,76(8) % 9/2+ | -0,87824(5) | -0,9360303 | -0,196(1) | 1,4897 | 0,00141 1,1547 |
*) bezogen auf 1H = 1,000
Strahlenschutz
Für den Umgang mit den Germanium-Radionukliden gelten gemäß Strahlenschutzverordnung (StrlSchV 2018) unter anderem folgende Werte (Spalten 1 bis 7):
| Nuklid | Freigrenzen | HRQ-Schwelle | OFK | Tochternuklide | Halbwertszeit | |
|---|---|---|---|---|---|---|
| Ge-66 | 106 Bq | 10 Bq/g | 2,3 Stunden | |||
| Ge-67 | 105 Bq | 10 Bq/g | 18,9 Minuten | |||
| Ge-68+ | 105 Bq | 0,1 Bq/g | 0,07 TBq | 1 Bq cm-2 | Ga-68 | 271,0 Tage |
| Ge-69 | 106 Bq | 10 Bq/g | 39,1 Stunden | |||
| Ge-71 | 108 Bq | 10000 Bq/g | 1000 TBq | 100 Bq cm-2 | 11,4 Tage | |
| Ge-75 | 106 Bq | 1000 Bq/g | 82,8 Minuten | |||
| Ge-77 | 105 Bq | 10 Bq/g | 0,06 TBq | 11,3 Stunden | ||
| Ge-78 | 106 Bq | 100 Bq/g | 88,0 Minuten | |||
(HRQ = Hochradioaktive Quellen; OFK = Oberflächenkontamination)
Kernisobare Nuklide des Germaniums
Zu den Germanium-Nukliden isobare Atomkerne befinden sich in der jeweiligen Tabellenzeile; Z = Ordnungszahl; A = Nukleonenzahl (Massenzahl).
| Z: | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh |
| 60 | 60Sc | 60Ti | 60V | 60Cr | 60Mn | 60Fe | 60Co | 60Ni | 60Cu | 60Zn | 60Ga | 60Ge | |||||||||||||
| 61 | 61Sc | 61Ti | 61V | 61Cr | 61Mn | 61Fe | 61Co | 61Ni | 61Cu | 61Zn | 61Ga | 61Ge | |||||||||||||
| 62 | 62Ti | 62V | 62Cr | 62Mn | 62Fe | 62Co | 62Ni | 62Cu | 62Zn | 62Ga | 62Ge | 62As | |||||||||||||
| 63 | 63Ti | 63V | 63Cr | 63Mn | 63Fe | 63Co | 63Ni | 63Cu | 63Zn | 63Ga | 63Ge | 63As | |||||||||||||
| 64 | 64V | 64Cr | 64Mn | 64Fe | 64Co | 64Ni | 64Cu | 64Zn | 64Ga | 64Ge | 64As | ||||||||||||||
| 65 | 65V | 65Cr | 65Mn | 65Fe | 65Co | 65Ni | 65Cu | 65Zn | 65Ga | 65Ge | 65As | 65Se | |||||||||||||
| 66 | 66V | 66Cr | 66Mn | 66Fe | 66Co | 66Ni | 66Cu | 66Zn | 66Ga | 66Ge | 66As | 66Se | 66Br | ||||||||||||
| 67 | 67Cr | 67Mn | 67Fe | 67Co | 67Ni | 67Cu | 67Zn | 67Ga | 67Ge | 67As | 67Se | 67Br | |||||||||||||
| 68 | 68Cr | 68Mn | 68Fe | 68Co | 68Ni | 68Cu | 68Zn | 68Ga | 68Ge | 68As | 68Se | 68Br | |||||||||||||
| 69 | 69Mn | 69Fe | 69Co | 69Ni | 69Cu | 69Zn | 69Ga | 69Ge | 69As | 69Se | 69Br | 69Kr | |||||||||||||
| 70 | 70Fe | 70Co | 70Ni | 70Cu | 70Zn | 70Ga | 70Ge | 70As | 70Se | 70Br | 70Kr | ||||||||||||||
| 71 | 71Fe | 71Co | 71Ni | 71Cu | 71Zn | 71Ga | 71Ge | 71As | 71Se | 71Br | 71Kr | 71Rb | |||||||||||||
| 72 | 72Fe | 72Co | 72Ni | 72Cu | 72Zn | 72Ga | 72Ge | 72As | 72Se | 72Br | 72Kr | 72Rb | |||||||||||||
| 73 | 73Co | 73Ni | 73Cu | 73Zn | 73Ga | 73Ge | 73As | 73Se | 73Br | 73Kr | 73Rb | 73Sr | |||||||||||||
| 74 | 74Co | 74Ni | 74Cu | 74Zn | 74Ga | 74Ge | 74As | 74Se | 74Br | 74Kr | 74Rb | 74Sr | |||||||||||||
| 75 | 75Co | 75Ni | 75Cu | 75Zn | 75Ga | 75Ge | 75As | 75Se | 75Br | 75Kr | 75Rb | 75Sr | |||||||||||||
| 76 | 76Co | 76Ni | 76Cu | 76Zn | 76Ga | 76Ge | 76As | 76Se | 76Br | 76Kr | 76Rb | 76Sr | 76Y | ||||||||||||
| 77 | 77Ni | 77Cu | 77Zn | 77Ga | 77Ge | 77As | 77Se | 77Br | 77Kr | 77Rb | 77Sr | 77Y | |||||||||||||
| 78 | 78Ni | 78Cu | 78Zn | 78Ga | 78Ge | 78As | 78Se | 78Br | 78Kr | 78Rb | 78Sr | 78Y | 78Zr | ||||||||||||
| 79 | 79Cu | 79Zn | 79Ga | 79Ge | 79As | 79Se | 79Br | 79Kr | 79Rb | 79Sr | 79Y | 79Zr | |||||||||||||
| 80 | 80Cu | 80Zn | 80Ga | 80Ge | 80As | 80Se | 80Br | 80Kr | 80Rb | 80Sr | 80Y | 80Zr | |||||||||||||
| 81 | 81Cu | 81Zn | 81Ga | 81Ge | 81As | 81Se | 81Br | 81Kr | 81Rb | 81Sr | 81Y | 81Zr | 81Nb | ||||||||||||
| 82 | 82Cu | 82Zn | 82Ga | 82Ge | 82As | 82Se | 82Br | 82Kr | 82Rb | 82Sr | 82Y | 82Zr | 82Nb | ||||||||||||
| 83 | 83Zn | 83Ga | 83Ge | 83As | 83Se | 83Br | 83Kr | 83Rb | 83Sr | 83Y | 83Zr | 83Nb | 83Mo | ||||||||||||
| 84 | 84Zn | 84Ga | 84Ge | 84As | 84Se | 84Br | 84Kr | 84Rb | 84Sr | 84Y | 84Zr | 84Nb | 84Mo | ||||||||||||
| 85 | 85Ga | 85Ge | 85As | 85Se | 85Br | 85Kr | 85Rb | 85Sr | 85Y | 85Zr | 85Nb | 85Mo | 85Tc | ||||||||||||
| 86 | 86Ga | 86Ge | 86As | 86Se | 86Br | 86Kr | 86Rb | 86Sr | 86Y | 86Zr | 86Nb | 86Mo | 86Tc | ||||||||||||
| 87 | 87Ga | 87Ge | 87As | 87Se | 87Br | 87Kr | 87Rb | 87Sr | 87Y | 87Zr | 87Nb | 87Mo | 87Tc | 87Ru | |||||||||||
| 88 | 88Ge | 88As | 88Se | 88Br | 88Kr | 88Rb | 88Sr | 88Y | 88Zr | 88Nb | 88Mo | 88Tc | 88Ru | ||||||||||||
| 89 | 89Ge | 89As | 89Se | 89Br | 89Kr | 89Rb | 89Sr | 89Y | 89Zr | 89Nb | 89Mo | 89Tc | 89Ru | 89Rh |
Kernisotone Nuklide des Germaniums
Die zu den Germanium-Kernen isotonen Nuklide befinden sich in der jeweiligen Tabellenzeile; N = Anzahl der Neutronen.
| 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 39Na | |||||||||||||||||||||||||||||
| 40Mg | |||||||||||||||||||||||||||||
| 41Al | 42Al | 43Al | |||||||||||||||||||||||||||
| 42Si | 43Si | 44Si | |||||||||||||||||||||||||||
| 43P | 44P | 45P | 46P | ||||||||||||||||||||||||||
| 44S | 45S | 46S | 47S | 48S | 49S | ||||||||||||||||||||||||
| 45Cl | 46Cl | 47Cl | 48Cl | 49Cl | 50Cl | 51Cl | |||||||||||||||||||||||
| 46Ar | 47Ar | 48Ar | 49Ar | 50Ar | 51Ar | 52Ar | 53Ar | ||||||||||||||||||||||
| 47K | 48K | 49K | 50K | 51K | 52K | 53K | 54K | 55K | 56K | ||||||||||||||||||||
| 48Ca | 49Ca | 50Ca | 51Ca | 52Ca | 53Ca | 54Ca | 55Ca | 56Ca | 57Ca | 58Ca | |||||||||||||||||||
| 49Sc | 50Sc | 51Sc | 52Sc | 53Sc | 54Sc | 55Sc | 56Sc | 57Sc | 58Sc | 59Sc | 60Sc | 61Sc | |||||||||||||||||
| 50Ti | 51Ti | 52Ti | 53Ti | 54Ti | 55Ti | 56Ti | 57Ti | 58Ti | 59Ti | 60Ti | 61Ti | 62Ti | 63Ti | ||||||||||||||||
| 51V | 52V | 53V | 54V | 55V | 56V | 57V | 58V | 59V | 60V | 61V | 62V | 63V | 64V | 65V | 66V | ||||||||||||||
| 52Cr | 53Cr | 54Cr | 55Cr | 56Cr | 57Cr | 58Cr | 59Cr | 60Cr | 61Cr | 62Cr | 63Cr | 64Cr | 65Cr | 66Cr | 67Cr | 68Cr | |||||||||||||
| 53Mn | 54Mn | 55Mn | 56Mn | 57Mn | 58Mn | 59Mn | 60Mn | 61Mn | 62Mn | 63Mn | 64Mn | 65Mn | 66Mn | 67Mn | 68Mn | 69Mn | |||||||||||||
| 54Fe | 55Fe | 56Fe | 57Fe | 58Fe | 59Fe | 60Fe | 61Fe | 62Fe | 63Fe | 64Fe | 65Fe | 66Fe | 67Fe | 68Fe | 69Fe | 70Fe | 71Fe | 72Fe | |||||||||||
| 55Co | 56Co | 57Co | 58Co | 59Co | 60Co | 61Co | 62Co | 63Co | 64Co | 65Co | 66Co | 67Co | 68Co | 69Co | 70Co | 71Co | 72Co | 73Co | 74Co | 75Co | 76Co | ||||||||
| 56Ni | 57Ni | 58Ni | 59Ni | 60Ni | 61Ni | 62Ni | 63Ni | 64Ni | 65Ni | 66Ni | 67Ni | 68Ni | 69Ni | 70Ni | 71Ni | 72Ni | 73Ni | 74Ni | 75Ni | 76Ni | 77Ni | 78Ni | |||||||
| 57Cu | 58Cu | 59Cu | 60Cu | 61Cu | 62Cu | 63Cu | 64Cu | 65Cu | 66Cu | 67Cu | 68Cu | 69Cu | 70Cu | 71Cu | 72Cu | 73Cu | 74Cu | 75Cu | 76Cu | 77Cu | 78Cu | 79Cu | 80Cu | 81Cu | 82Cu | ||||
| 58Zn | 59Zn | 60Zn | 61Zn | 62Zn | 63Zn | 64Zn | 65Zn | 66Zn | 67Zn | 68Zn | 69Zn | 70Zn | 71Zn | 72Zn | 73Zn | 74Zn | 75Zn | 76Zn | 77Zn | 78Zn | 79Zn | 80Zn | 81Zn | 82Zn | 83Zn | 84Zn | |||
| 59Ga | 60Ga | 61Ga | 62Ga | 63Ga | 64Ga | 65Ga | 66Ga | 67Ga | 68Ga | 69Ga | 70Ga | 71Ga | 72Ga | 73Ga | 74Ga | 75Ga | 76Ga | 77Ga | 78Ga | 79Ga | 80Ga | 81Ga | 82Ga | 83Ga | 84Ga | 85Ga | 86Ga | 87Ga | |
| 60Ge | 61Ge | 62Ge | 63Ge | 64Ge | 65Ge | 66Ge | 67Ge | 68Ge | 69Ge | 70Ge | 71Ge | 72Ge | 73Ge | 74Ge | 75Ge | 76Ge | 77Ge | 78Ge | 79Ge | 80Ge | 81Ge | 82Ge | 83Ge | 84Ge | 85Ge | 86Ge | 87Ge | 88Ge | 89Ge |
| 62As | 63As | 64As | 65As | 66As | 67As | 68As | 69As | 70As | 71As | 72As | 73As | 74As | 75As | 76As | 77As | 78As | 79As | 80As | 81As | 82As | 83As | 84As | 85As | 86As | 87As | 88As | 89As | 90As | |
| 65Se | 66Se | 67Se | 68Se | 69Se | 70Se | 71Se | 72Se | 73Se | 74Se | 75Se | 76Se | 77Se | 78Se | 79Se | 80Se | 81Se | 82Se | 83Se | 84Se | 85Se | 86Se | 87Se | 88Se | 89Se | 90Se | 91Se | |||
| 66Br | 67Br | 68Br | 69Br | 70Br | 71Br | 72Br | 73Br | 74Br | 75Br | 76Br | 77Br | 78Br | 79Br | 80Br | 81Br | 82Br | 83Br | 84Br | 85Br | 86Br | 87Br | 88Br | 89Br | 90Br | 91Br | 92Br | |||
| 69Kr | 70Kr | 71Kr | 72Kr | 73Kr | 74Kr | 75Kr | 76Kr | 77Kr | 78Kr | 79Kr | 80Kr | 81Kr | 82Kr | 83Kr | 84Kr | 85Kr | 86Kr | 87Kr | 88Kr | 89Kr | 90Kr | 91Kr | 92Kr | 93Kr | |||||
| 71Rb | 72Rb | 73Rb | 74Rb | 75Rb | 76Rb | 77Rb | 78Rb | 79Rb | 80Rb | 81Rb | 82Rb | 83Rb | 84Rb | 85Rb | 86Rb | 87Rb | 88Rb | 89Rb | 90Rb | 91Rb | 92Rb | 93Rb | 94Rb | ||||||
| 73Sr | 74Sr | 75Sr | 76Sr | 77Sr | 78Sr | 79Sr | 80Sr | 81Sr | 82Sr | 83Sr | 84Sr | 85Sr | 86Sr | 87Sr | 88Sr | 89Sr | 90Sr | 91Sr | 92Sr | 93Sr | 94Sr | 95Sr | |||||||
| 76Y | 77Y | 78Y | 79Y | 80Y | 81Y | 82Y | 83Y | 84Y | 85Y | 86Y | 87Y | 88Y | 89Y | 90Y | 91Y | 92Y | 93Y | 94Y | 95Y | 96Y | |||||||||
| 78Zr | 79Zr | 80Zr | 81Zr | 82Zr | 83Zr | 84Zr | 85Zr | 86Zr | 87Zr | 88Zr | 89Zr | 90Zr | 91Zr | 92Zr | 93Zr | 94Zr | 95Zr | 96Zr | 97Zr | ||||||||||
| 81Nb | 82Nb | 83Nb | 84Nb | 85Nb | 86Nb | 87Nb | 88Nb | 89Nb | 90Nb | 91Nb | 92Nb | 93Nb | 94Nb | 95Nb | 96Nb | 97Nb | 98Nb | ||||||||||||
| 83Mo | 84Mo | 85Mo | 86Mo | 87Mo | 88Mo | 89Mo | 90Mo | 91Mo | 92Mo | 93Mo | 94Mo | 95Mo | 96Mo | 97Mo | 98Mo | 99Mo | |||||||||||||
| 85Tc | 86Tc | 87Tc | 88Tc | 89Tc | 90Tc | 91Tc | 92Tc | 93Tc | 94Tc | 95Tc | 96Tc | 97Tc | 98Tc | 99Tc | 100Tc | ||||||||||||||
| 87Ru | 88Ru | 89Ru | 90Ru | 91Ru | 92Ru | 93Ru | 94Ru | 95Ru | 96Ru | 97Ru | 98Ru | 99Ru | 100Ru | 101Ru | |||||||||||||||
| 89Rh | 90Rh | 91Rh | 92Rh | 93Rh | 94Rh | 95Rh | 96Rh | 97Rh | 98Rh | 99Rh | 100Rh | 101Rh | 102Rh | ||||||||||||||||
| 91Pd | 92Pd | 93Pd | 94Pd | 95Pd | 96Pd | 97Pd | 98Pd | 99Pd | 100Pd | 101Pd | 102Pd | 103Pd | |||||||||||||||||
| 93Ag | 94Ag | 95Ag | 96Ag | 97Ag | 98Ag | 99Ag | 100Ag | 101Ag | 102Ag | 103Ag | 104Ag | ||||||||||||||||||
| 95Cd | 96Cd | 97Cd | 98Cd | 99Cd | 100Cd | 101Cd | 102Cd | 103Cd | 104Cd | 105Cd | |||||||||||||||||||
| 97In | 98In | 99In | 100In | 101In | 102In | 103In | 104In | 105In | 106In | ||||||||||||||||||||
| 99Sn | 100Sn | 101Sn | 102Sn | 103Sn | 104Sn | 105Sn | 106Sn | 107Sn | |||||||||||||||||||||
| 103Sb | 104Sb | 105Sb | 106Sb | 107Sb | 108Sb | ||||||||||||||||||||||||
| 105Te | 106Te | 107Te | 108Te | 109Te | |||||||||||||||||||||||||
| 108I | 109I | 110I | |||||||||||||||||||||||||||
| 109Xe | 110Xe | 111Xe | |||||||||||||||||||||||||||
| 112Cs |
Literatur und Hinweise
Eigenschaften der Germanium-Isotope
[1] - NuDat: National Nuclear Data Center, Brookhaven National Laboratory, based on ENSDF and the Nuclear Wallet Cards.
[2] - G. Audi et. al.: The NUBASE evaluation of nuclear and decay properties. Nuclear Physics, (2003), DOI 10.1016/j.nuclphysa.2003.11.001.
[3] - Live Chart of Nuclides. Nuclear structure and decay data.
Germanium: Kernmagnetische Eigenschaften - 73Ge-NMR
[4] - N. J. Stone: Table of nuclear magnetic dipole and electric quadrupole moments. Atomic Data and Nuclear Data Tables, (2005), DOI 10.1016/j.adt.2005.04.001.
[5] - Pekka Pyykkö: Year-2008 nuclear quadrupole moments. Molecular Physics, (2008), DOI 10.1080/00268970802018367.
[6] - Pekka Pyykkö: Year-2017 nuclear quadrupole moments. Molecular Physics, (2018), DOI 10.1080/00268976.2018.1426131.
[7] - N. J. Stone: Table of recommended nuclear magnetic dipole moments. IAEA, (2019).
Weitere Quellen:
[8] - Isotopenhäufigkeiten, Atommassen und Isotopenmassen: Siehe unter dem jeweiligen Stichwort.
[9] - A.M. Bakalyarov, A.Ya. Balysh, S.T. Belyaev, V.I. Lebedev, S.V. Zhukov:
Results of the experiment on investigation of Germanium-76 double beta decay.
In: Experimental data of Heidelberg-Moscow collaboration November 1995 - August 2001, (2003), DOI https://arxiv.org/abs/hep-ex/0309016.
[10] - J. L. Gross, M. Thoennessen:
Discovery of gallium, germanium, lutetium, and hafnium isotopes.
In: Atomic Data and Nuclear Data Tables, (2012), DOI 10.1016/j.adt.2011.09.004.
Kategorie: Chemische Elemente
Letzte Änderung am 12.12.2022.
Permalink: https://www.internetchemie.info/chemische-elemente/germanium-isotope.php.
© 1996 - 2026 Internetchemie ChemLin