Unter Tellur-Isotope werden alle Atomkerne des chemischen Elements Tellur zusammengefasst; diese bestehen allesamt aus einem Atomkern mit 52 Protonen und im ungeladenen Zustand aus 52 Elektronen. Der Unterschied zwischen den einzelnen Tellur-Isotopen beruht auf der Anzahl der Neutronen im Kern.
Natürlich auftretende Tellur-Isotope
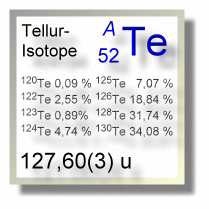
Natürliche irdische Tellur-Vorkommen setzem sich aus acht Isotopen zusammen, von denen fünf als stabil gelten und drei mit sehr langen Halbwertszeiten unter Abgabe leichter radioaktiver Strahlung zerfallen. Den größten Anteil mit 32 bzw. 34 % liefern die beiden unter doppelten Elektroneneinfang zerfallenden Tellurisotope Te-128 und Te-130.
Der Anteil der schwereren Tellurisotope führt dazu, dass die mittlere relative Atommasse mit 127,60 u über der des schwereren Elements Iod (126,90 u) liegt.
Technische Anwendungen für die einzelnen Tellurisotope gibt es nicht.
| Atommasse Ar | Anteil | Halbwertszeit | Spin | |
| Tellur Isotopengemisch | 127,60 u | 100 % | ||
| Isotop 120Te | 119,90406(2) u | 0,09(1) % | stabil | 0+ |
| Isotop 122Te | 121,90304(1) u | 2,55(12) % | stabil | 0+ |
| Isotop 123Te | 122,90427(1) u | 0,89(3) % | 9,2 × 1016 Jahre | 1/2+ |
| Isotop 124Te | 123,90282(1) u | 4,74(14) % | stabil | 0+ |
| Isotop 125Te | 124,9044312(15) u | 7,07(15) % | stabil | 1/2+ |
| Isotop 126Te | 125,90331(1) u | 18,84(25) % | stabil | 0+ |
| Isotop 128Te | 127,904461(6) u | 31,74(8) % | 7,7(4) × 1024 Jahre | 0+ |
| Isotop 130Te | 129,90622275(8) u | 34,08(62) % | 0,79 × 1021 Jahre | 0+ |
Neben den natürlichen Te-Isotopen kennt man etwa 30 weitere instabile Radionuklide des Tellurs und mehrere Kernisomere.
Isotopentabelle: Tellur
| Isotop Nuklid | Z | A | N | Name | Atommasse [Kernmasse] {Massenüberschuss} | Spin I (h/2π) | μ | A-Nuk |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| 105Te | 52 | 105 | 53 | Tellur-105 | 104,94330(32) u [104,9147756 u] {-52,81571 MeV} | (5/2+) | ||
| 106Te | 52 | 106 | 54 | Tellur-106 | 105,93750(11) u [105,9089756 u] {-58,21838 MeV} | 0+ | 110Xe | |
| 107Te | 52 | 107 | 55 | Tellur-107 | 106,93501(8) u [106,9064856 u] {-60,5378 MeV} | 111Xe | ||
| 108Te | 52 | 108 | 56 | Tellur-108 | 107,92938(6) u [107,9008556 u] {-65,78211 MeV} | 0+ | 112Xe | |
| 109Te | 52 | 109 | 57 | Tellur-109 | 108,927305(5) u [108,8987806 u] {-67,71496 MeV} | (5/2+) | 113Xe | |
| 110Te | 52 | 110 | 58 | Tellur-110 | 109,922458(7) u [109,8939336 u] {-72,22991 MeV} | 0+ | ||
| 111Te | 52 | 111 | 59 | Tellur-111 | 110,921001(7) u [110,8924766 u] {-73,58709 MeV} | (5/2)+ | ||
| 112Te | 52 | 112 | 60 | Tellur-112 | 111,916728(9) u [111,8882036 u] {-77,56737 MeV} | 0+ | 112I | |
| 113Te | 52 | 113 | 61 | Tellur-113 | 112,91589(3) u [112,8873656 u] {-78,34796 MeV} | (7/2+) | 113I | |
| 114Te | 52 | 114 | 62 | Tellur-114 | 113,91209(3) u [113,8835656 u] {-81,88764 MeV} | 0+ | 114I | |
| 115Te | 52 | 115 | 63 | Tellur-115 | 114,91190(3) u [114,8833756 u] {-82,06462 MeV} | 7/2+ | 115I | |
| 115mTe | 52 | 115 | 63 | Tellur-115m | 114,91190(3) u [114,8833756 u] {-82,06462 MeV} | (1/2)+ | ||
| 116Te | 52 | 116 | 64 | Tellur-116 | 115,90846(3) u [115,8799356 u] {-85,26896 MeV} | 0+ | 116I | |
| 117Te | 52 | 117 | 65 | Tellur-117 | 116,908646(14) u [116,8801216 u] {-85,0957 MeV} | 1/2+ | 117I | |
| 117mTe | 52 | 117 | 65 | Tellur-117m | 116,908646(14) u [116,8801216 u] {-85,0957 MeV} | (11/2-) | ||
| 118Te | 52 | 118 | 66 | Tellur-118 | 117,905854(20) u [117,8773296 u] {-87,69643 MeV} | 0+ | 118I | |
| 119Te | 52 | 119 | 67 | Tellur-119 | 118,906407(9) u [118,8778826 u] {-87,18132 MeV} | 1/2+ | 0,25(5) | |
| 119mTe | 52 | 119 | 67 | Tellur-119m | 118,906407(9) u [118,8778826 u] {-87,18132 MeV} | 11/2- | 0,894(6) | |
| 120Te | 52 | 120 | 68 | Tellur-120 | 119,90406(2) u [119,8755356 u] {-89,36753 MeV} | 0+ | 120I | |
| 121Te | 52 | 121 | 69 | Tellur-121 | 120,904945(28) u [120,8764206 u] {-88,54316 MeV} | 1/2+ | 121I | |
| 121mTe | 52 | 121 | 69 | Tellur-121m | 120,904945(28) u [120,8764206 u] {-88,54316 MeV} | 11/2- | 0,895(10) | |
| 122Te | 52 | 122 | 70 | Tellur-122 | 121,90304(1) u [121,8745156 u] {-90,31766 MeV} | 0+ | ||
| 123Te | 52 | 123 | 71 | Tellur-123 | 122,90427(1) u [122,8757456 u] {-89,17192 MeV} | 1/2+ | -0,7358(3) | 123I |
| 123mTe | 52 | 123 | 71 | Tellur-123m | 122,90427(1) u [122,8757456 u] {-89,17192 MeV} | 11/2- | - 0,927(8) | |
| 124Te | 52 | 124 | 72 | Tellur-124 | 123,90282(1) u [123,8742956 u] {-90,52259 MeV} | 0+ | 124I 124Sb | |
| 125Te | 52 | 125 | 73 | Tellur-125 | 124,9044312(15) u [124,8759068 u] {-89,02176 MeV} | 1/2+ | -0,8885051(4) | 125I 125Sb |
| 125mTe | 52 | 125 | 73 | Tellur-125m | 124,9044312(15) u [124,8759068 u] {-89,02176 MeV} | 11/2- | - 0,985(6) | |
| 126Te | 52 | 126 | 74 | Tellur-126 | 125,90331(1) u [125,8747856 u] {-90,06615 MeV} | 0+ | 126I 126Sb | |
| 127Te | 52 | 127 | 75 | Tellur-127 | 126,9052257(16) u [126,8767013 u] {-88,28169 MeV} | 3/2+ | 0,635(4) | 127Sb |
| 127mTe | 52 | 127 | 75 | Tellur-127m | 126,9052257(16) u [126,8767013 u] {-88,28169 MeV} | 11/2- | - 1,041(6) | |
| 128Te | 52 | 128 | 76 | Tellur-128 | 127,904461(6) u [127,8759366 u] {-88,99401 MeV} | 0+ | 128Sb 128I | |
| 129Te | 52 | 129 | 77 | Tellur-129 | 128,9065965(9) u [128,8780721 u] {-87,0048 MeV} | 3/2+ | 0,702(4) | 129Sb |
| 129mTe | 52 | 129 | 77 | Tellur-129m | 128,9065965(9) u [128,8780721 u] {-87,0048 MeV} | 11/2- | - 1,091(7) | |
| 130Te | 52 | 130 | 78 | Tellur-130 | 129,90622275(8) u [129,8776984 u] {-87,35295 MeV} | 0+ | 130Sb | |
| 131Te | 52 | 131 | 79 | Tellur-131 | 130,90852221(7) u [130,8799978 u] {-85,21101 MeV} | 3/2+ | 0,696(9) | 131Sb |
| 131mTe | 52 | 131 | 79 | Tellur-131m | 130,90852221(6) u [130,8799978 u] {-85,21101 MeV} | 11/2- | -1,123(7) | |
| 131m2Te | 52 | 131 | 79 | Tellur-131m2 | 130,90852221(6) u [130,8799978 u] {-85,21101 MeV} | (23/2+) | ||
| 131m+Te | 52 | 131 | 79 | Tellur-131m+ | ||||
| 132Te | 52 | 132 | 80 | Tellur-132 | 131,908547(4) u [131,8800226 u] {-85,18792 MeV} | 0+ | 132Sb 252Cf | |
| 133Te | 52 | 133 | 81 | Tellur-133 | 132,9109633(22) u [132,8824389 u] {-82,93715 MeV} | (3/2+) | 0,85(2) | 133Sb |
| 133mTe | 52 | 133 | 81 | Tellur-133m | 132,9109633(22) u [132,8824389 u] {-82,93715 MeV} | (11/2-) | (-) 1,129(7) | |
| 133m+Te | 52 | 133 | 81 | Tellur-133m+ | ||||
| 134Te | 52 | 134 | 82 | Tellur-134 | 133,9113964(29) u [133,882872 u] {-82,53372 MeV} | 0+ | 134Sb 235Sb 248Cm | |
| 135Te | 52 | 135 | 83 | Tellur-135 | 134,9165547(18) u [134,8880303 u] {-77,7288 MeV} | (7/2-) | - 0,69(5) | 135Sb 136Sb 248Cm 252Cf |
| 136Te | 52 | 136 | 84 | Tellur-136 | 135,9201012(24) u [135,8915768 u] {-74,42525 MeV} | 0+ | 136Sb 248Cm 252Cf | |
| 137Te | 52 | 137 | 85 | Tellur-137 | 136,9255994(23) u [136,897075 u] {-69,30371 MeV} | (7/2-) | 248Cm | |
| 138Te | 52 | 138 | 86 | Tellur-138 | 137,929472(4) u [137,9009476 u] {-65,69641 MeV} | 0+ | 248Cm | |
| 139Te | 52 | 139 | 87 | Tellur-139 | 138,935367(4) u [138,9068426 u] {-60,20525 MeV} | (7/2-) | 248Cm | |
| 140Te | 52 | 140 | 88 | Tellur-140 | 139,93926(7) u [139,9107356 u] {-56,57895 MeV} | 0+ | ||
| 141Te | 52 | 141 | 89 | Tellur-141 | ||||
| 142Te | 52 | 142 | 90 | Tellur-142 | 141,95022(54) u [141,9216956 u] {-46,36977 MeV} | 0+ | ||
| 143Te | 52 | 143 | 91 | Tellur-143 | 142,95676(54) u [142,9282356 u] {-40,2778 MeV} |
| Isotop | Zerfall (radioaktiver Zerfall) | AE | Mehr | |||
|---|---|---|---|---|---|---|
| Halbwertszeit | Zerfallsart | Anteil | Energie | Info | ||
| 1 | 10 | 11 | 12 | 13 | 14 | 15 |
| Te-105 | 0,62(7) μs | α zu 101Sn | ca. 100 % | 5,069(3) MeV | AL | |
| Te-106 | 70(17) μs | α zu 102Sn | 100 % | 4,290(9) MeV | AL | |
| Te-107 | 3,1(1) ms | α zu 103Sn EE/β+ zu 107Sb | 70(30) % 30(30) % | 4,008(5) MeV 10,11(7) MeV | AL | |
| Te-108 | 2,1(1) s | α zu 104Sn EE/β+ zu 108Sb β+, p zu 107Sb | 49(9) % 51(4) % 2,4(10) % | 3,420(8) MeV 6,664(8) | AL | |
| Te-109 | 4,4(2) s | α zu 105Sn EE/β+ zu 109Sb β+, p zu 108Sb β+, α zu 105In | 3,9(13) % 96,1(13) % 9,4(31) % < 0,005 % | 3,198(6) MeV 8,536(7) MeV | AL | |
| Te-110 | 18,6(8) s | EE/β+ zu 110Sb α zu 106Sn | ca. 100 % 0,00067 % | 5,220(9) MeV 2,699(8) MeV | AL | |
| Te-111 | 19,3(4) s | EE/β+ zu 111Sb β+, p zu 110Sb | 100 % ? | 7,249(11) MeV | AL | |
| Te-112 | 2,0(2) Minuten | EE/β+ zu 112Sb | 100 % | 4,031(20) MeV | AL | |
| Te-113 | 1,7(2) Minuten | EE/β+ zu 113Sb | 100 % | 6,07(3) MeV | AL | |
| Te-114 | 15,2(7) Minuten | EE/β+ zu 114Sb | 100 % | 2,61(4) MeV | AL | |
| Te-115 | 5,8(2) Minuten | EE/β+ zu 115Sb | 100 % | 4,94(3) MeV | AL | |
| Te-115m | 6,7(4) Minuten | EE/β+ zu 115Sb Iso zu 115Te | < 100 % ? | |||
| Te-116 | 2,49(4) Stunden | EE/β+ zu 116Sb | 100 % | 1,553(28) MeV | AL | |
| Te-117 | 62(2) Minuten | EE/β+ zu 117Sb β+ zu 117Sb | 75 % 25(1) % | 3,544(16) MeV ? | AL | |
| Te-117m | 103(3) ms | Iso zu 117Te | 100 % | 296,1 keV | ||
| Te-118 | 6,00(2) Tage | EE zu 118Sb | 100 % | 0,300(19) MeV | AL | |
| Te-119 | 16,05(5) Stunden | EE/β+ zu 119Sb β+ zu 119Sb | 97,04 % 2,06 % | 2,293(11) MeV ? | AL | |
| Te-119m | 4,70(4) Tage | EE/β+ zu 119Sb β+ zu 119Sb Iso zu 119Te | > 99 % 0,41(4) % < 0,008 % | 260,96(5) keV | ||
| Te-120 | stabil | AL | ||||
| Te-121 | 19,17(4) Tage | EE/β+ zu 121Sb | 100 % | 1,056(26) MeV | AL | |
| Te-121m | 164,2(8) Tage | EE/β+ zu 121Sb Iso zu 121Te | 11,4(11) % 88,6(11) % | 293,974(22) keV | ||
| Te-122 | stabil | AL | ||||
| Te-123 | 9,2 × 1016 Jahre | EE zu 123Sb | 100 % | 0,0519(21) MeV | AL | |
| Te-123m | 119,2(1) Tage | Iso zu 123Te | 100 % | 247,47(4) keV | ||
| Te-124 | stabil | AL | ||||
| Te-125 | stabil | AL | ||||
| Te-125m | 57,40(15) Tage | Iso zu 125Te | 100 % | 144,775(8) keV | ||
| Te-126 | stabil | AL | ||||
| Te-127 | 9,35(7) Stunden | β- zu 127I | 100 % | 0,702(4) MeV | AL | |
| Te-127m | 106,1(7) Tage | β- zu 127I Iso zu 127Te | 2,4(2) % 97,6(2) % | 88,23(7) keV | ||
| Te-128 | 7,7(4) × 1024 Jahre | 2 β- zu 128Xe | 100 % | AL | ||
| Te-129 | 69,6(3) Minuten | β- zu 129I | 100 % | 1,502(3) MeV | AL | |
| Te-129m | 33,6(1) Tage | β- zu 129I Iso zu 129Te | 36(7) % 64(7) % | 105,51(3) keV | ||
| Te-130 | 0,79 × 1021 Jahre | 2 β- zu 130Xe | 100 % | AL | ||
| Te-131 | 25,0(1) Minuten | β- zu 131I | 100 % | 2,2317(6) MeV | AL | |
| Te-131m | 33,25(25) Stunden | β- zu 131I Iso zu 131Te | 74,1(5) % 25,9(5) % | 182,258(18) keV | ||
| Te-131m2 | 93(12) ms | Iso zu 131Te | 100 % | 1940,0(4) keV | ||
| Te-131m+ | ||||||
| Te-132 | 3,204(13) Tage | β- zu 132I | 100 % | 0,515(3) MeV | AL | |
| Te-133 | 12,5(3) Minuten | β- zu 133I | 100 % | 2,921(7) MeV | AL | |
| Te-133m | 55,4(4) Minuten | β- zu 133I Iso zu 133Te | 83,5(20) % 16,5(20) % | 334,26(4) keV | ||
| Te-133m+ | ||||||
| Te-134 | 41,8(8) Minuten | β- zu 134I | 100 % | 1,510(5) MeV | AL | |
| Te-135 | 19,0(2) s | β- zu 135I | 100 % | 6,0504(27) MeV | AL | |
| Te-136 | 17,63(8) s | β- zu 136I β-, n zu 135I | 98,69 % 1,31 % | 5,120(14) MeV 1,283(3) MeV | AL | |
| Te-137 | 2,49(5) s | β- zu 137I β-, n zu 136I | 97,01 % 2,99 % | 7,053(9) MeV 2,170(14) MeV | AL | |
| Te-138 | 1,4(4) s | β- zu 138I β-, n zu 137I | 93,7 % 6,3 % | 6,284(7) MeV 2,589(9) MeV | AL | |
| Te-139 | 1,6(3) s | β- zu 129I β-, n zu 128I | 100 % ? | 8,266(5) MeV 3,704(7) MeV | AL | |
| Te-140 | β- zu 140I β-, n zu 139I | 100 % ? | 7,03(6) MeV 3,82(6) MeV | AL | ||
| Te-141 | - ungelistet - | AL | ||||
| Te-142 | AL | |||||
| Te-143 | 408 ns | β- zu 143I β-, n zu 142I β-, 2n zu 141I | ? ? ? | 10,35(54) MeV 6,42(63) MeV | AL | |
Erläuterungen zu den einzelnen Spalten:
1 - Symbol mit Nukleonenzahl.
2 - Z = Anzahl der Protonen (Ordnungszahl).
3 - Massenzahl A.
4 - N = Anzahl der Neutronen.
5 - Bezeichnung des Tellur-Isotops; gegebenenfalls Trivialnamen.
6 - Relative Atommasse des Tellur-Isotops (Isotopenmasse inklusive Elektronen) und in eckigen Klammern die Masse des Atomkerns (Kernmasse, Nuklidmasse ohne Elektronen), jeweils bezogen auf 12C = 12,00000 [2]. Zusätzlich ist der Massenüberschuss (Massenexzess) in MeV angegeben.
7 - Kernspin I, Einheit: h/2π.
8 - Kernmagnetisches Moment μmag.
9 - Ausgangsnuklide: Mögliche, angenommene oder tatsächliche Ausgangs-Nuklide (Mutternuklide, Elternnuklide). Die entsprechenden Zerfalls-Modi sind gegebenenfalls bei den Daten des jeweiligen Ausgangsnuklids zu finden.
10 - Zerfall: Halbwertszeiten des Tellur-Isotops mit a = Jahre; ; d = Tage; h = Stunden; min = Minuten; s = Sekunden.
11 - Zerfall: Zerfallsart in die jeweiligen Tochternuklide mit n = Neutronenemission; p = Protonenemission; α = Alpha-Zerfall; ß- = Beta-Minus-Zerfall unter Elektronenemission; EE = Elektroneneinfang; ß+ = Positronenemission; ε = ß+ und/oder EE; Iso = Isomerieübergang; CZ = Cluster-Zerfall; SZ = Spontanzerfall.
12 - Zerfall: Zerfallsanteil in Prozent (%).
13 - Zerfall: Zerfallsenergie; Partikelenergie bezogen auf Zerfallsart.
14 - AE = Anregungsenergie für metastabile Kerne.
15 - Sonstige Informationen und Hinweise: AL = Weitere Niveaus, so genannte Adopted Levels (Verlinkung auf externe Daten [1]).
Sonstige:
()- Eingeklammerte Ziffern: Unsicherheit zur Darstellung der Streubreite des angegebenen Wertes.
~ - Theoretische Werte oder systematische Trends.
- ungelistet-: Nuklide, die in der Literatur bereits erwänhnt wurden, aber aus irgendwelchen Gründen in den aktuellen Nuklidtabellen nicht mehr zu finden sind, weil sich deren Entdeckung z. B. nicht bestätigt hat.
NMR-aktive Tellur-Nuklide
| Nuklid Anteil Spin I | Kernmagnetisches Moment μ/μN | Gyromagnetisches Verhältnis 107 rad T-1 s-1 | Quadrupol- Moment Q [barn] | Resonanz- Frequenz v0 bei 1 T | Relative Empfindlichkeit H0 = const. v0 = const. * |
|---|---|---|---|---|---|
| 123Te 0,89(3) % 1/2+ | -0,7358(3) | 7,0576 | 11,2349 | 0,01837 0,2639 | |
| 125Te 7,07(15) % 1/2+ | -0,8885051(4) | 8,5087 | 13,5446 | 0,03219 0,3181 |
*) bezogen auf 1H = 1,000
Strahlenschutz
Für den Umgang mit den Tellur-Radionukliden gelten gemäß Strahlenschutzverordnung (StrlSchV 2018) unter anderem folgende Werte (Spalten 1 bis 7):
| Nuklid | Freigrenzen | HRQ-Schwelle | OFK | Tochternuklide | Halbwertszeit | |
|---|---|---|---|---|---|---|
| Te-116+ | 107 Bq | 100 Bq/g | 2,5 Stunden | |||
| Te-121 | 106 Bq | 10 Bq/g | 0,1 TBq | 19,2 Tage | ||
| Te-121m | 106 Bq | 1 Bq/g | 0,1 TBq | 154,0 Tage | ||
| Te-123 | 106 Bq | 0,1 Bq/g | > 9,2 × 1016 Jahre | |||
| Te-123m | 107 Bq | 100 Bq/g | 8 × 1010 Bq | 10 Bq/cm2 | 119,7 Tage | |
| Te-125m | 107 Bq | 1000 Bq/g | 10 TBq | 100 Bq cm-2 | 57,4 Tage | |
| Te-127 | 106 Bq | 1000 Bq/g | 10 TBq | 100 Bq cm-2 | 9,4 Stunden | |
| Te-127m+ | 107 Bq | 10 Bq/g | 3 TBq | 100 Bq cm-2 | Te-127 | 109,0 Tage |
| Te-129 | 106 Bq | 100 Bq/g | 1 TBq | 10 Bq cm-2 | 69,6 Minuten | |
| Te-129m+ | 106 Bq | 10 Bq/g | 1 TBq | 10 Bq cm-2 | Te-129 | 33,6 Tage |
| Te-131 | 105 Bq | 100 Bq/g | 10 Bq cm-2 | 25,0 Minuten | ||
| Te-131m+ | 106 Bq | 10 Bq/g | 0,04 TBq | 1 Bq cm-2 | 30,0 Stunden | |
| Te-132 | 107 Bq | 100 Bq/g | 5 × 109 Bq | 1 Bq/cm2 | 76,3 Stunden | |
| Te-133 | 105 Bq | 10 Bq/g | 1 Bq cm-2 | 12,5 Minuten | ||
| Te-133m+ | 105 Bq | 10 Bq/g | 1 Bq cm-2 | 55,4 Minuten | ||
| Te-134 | 106 Bq | 10 Bq/g | 1 Bq cm-2 | 41,8 Minuten | ||
(HRQ = Hochradioaktive Quellen; OFK = Oberflächenkontamination)
Kernisobare Nuklide des Tellurs
Zu den Tellur-Nukliden isobare Atomkerne befinden sich in der jeweiligen Tabellenzeile; Z = Ordnungszahl; A = Nukleonenzahl (Massenzahl).
| Z: | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er |
| 105 | 105Sr | 105Y | 105Zr | 105Nb | 105Mo | 105Tc | 105Ru | 105Rh | 105Pd | 105Ag | 105Cd | 105In | 105Sn | 105Sb | 105Te | ||||||||||||||||
| 106 | 106Sr | 106Y | 106Zr | 106Nb | 106Mo | 106Tc | 106Ru | 106Rh | 106Pd | 106Ag | 106Cd | 106In | 106Sn | 106Sb | 106Te | ||||||||||||||||
| 107 | 107Sr | 107Y | 107Zr | 107Nb | 107Mo | 107Tc | 107Ru | 107Rh | 107Pd | 107Ag | 107Cd | 107In | 107Sn | 107Sb | 107Te | ||||||||||||||||
| 108 | 108Y | 108Zr | 108Nb | 108Mo | 108Tc | 108Ru | 108Rh | 108Pd | 108Ag | 108Cd | 108In | 108Sn | 108Sb | 108Te | 108I | ||||||||||||||||
| 109 | 109Y | 109Zr | 109Nb | 109Mo | 109Tc | 109Ru | 109Rh | 109Pd | 109Ag | 109Cd | 109In | 109Sn | 109Sb | 109Te | 109I | 109Xe | |||||||||||||||
| 110 | 110Zr | 110Nb | 110Mo | 110Tc | 110Ru | 110Rh | 110Pd | 110Ag | 110Cd | 110In | 110Sn | 110Sb | 110Te | 110I | 110Xe | ||||||||||||||||
| 111 | 111Zr | 111Nb | 111Mo | 111Tc | 111Ru | 111Rh | 111Pd | 111Ag | 111Cd | 111In | 111Sn | 111Sb | 111Te | 111I | 111Xe | ||||||||||||||||
| 112 | 112Zr | 112Nb | 112Mo | 112Tc | 112Ru | 112Rh | 112Pd | 112Ag | 112Cd | 112In | 112Sn | 112Sb | 112Te | 112I | 112Xe | 112Cs | |||||||||||||||
| 113 | 113Nb | 113Mo | 113Tc | 113Ru | 113Rh | 113Pd | 113Ag | 113Cd | 113In | 113Sn | 113Sb | 113Te | 113I | 113Xe | 113Cs | ||||||||||||||||
| 114 | 114Nb | 114Mo | 114Tc | 114Ru | 114Rh | 114Pd | 114Ag | 114Cd | 114In | 114Sn | 114Sb | 114Te | 114I | 114Xe | 114Cs | 114Ba | |||||||||||||||
| 115 | 115Nb | 115Mo | 115Tc | 115Ru | 115Rh | 115Pd | 115Ag | 115Cd | 115In | 115Sn | 115Sb | 115Te | 115I | 115Xe | 115Cs | 115Ba | |||||||||||||||
| 116 | 116Mo | 116Tc | 116Ru | 116Rh | 116Pd | 116Ag | 116Cd | 116In | 116Sn | 116Sb | 116Te | 116I | 116Xe | 116Cs | 116Ba | 116La | |||||||||||||||
| 117 | 117Mo | 117Tc | 117Ru | 117Rh | 117Pd | 117Ag | 117Cd | 117In | 117Sn | 117Sb | 117Te | 117I | 117Xe | 117Cs | 117Ba | 117La | |||||||||||||||
| 118 | 118Tc | 118Ru | 118Rh | 118Pd | 118Ag | 118Cd | 118In | 118Sn | 118Sb | 118Te | 118I | 118Xe | 118Cs | 118Ba | 118La | ||||||||||||||||
| 119 | 119Tc | 119Ru | 119Rh | 119Pd | 119Ag | 119Cd | 119In | 119Sn | 119Sb | 119Te | 119I | 119Xe | 119Cs | 119Ba | 119La | 119Ce | |||||||||||||||
| 120 | 120Tc | 120Ru | 120Rh | 120Pd | 120Ag | 120Cd | 120In | 120Sn | 120Sb | 120Te | 120I | 120Xe | 120Cs | 120Ba | 120La | 120Ce | |||||||||||||||
| 121 | 121Ru | 121Rh | 121Pd | 121Ag | 121Cd | 121In | 121Sn | 121Sb | 121Te | 121I | 121Xe | 121Cs | 121Ba | 121La | 121Ce | 121Pr | |||||||||||||||
| 122 | 122Ru | 122Rh | 122Pd | 122Ag | 122Cd | 122In | 122Sn | 122Sb | 122Te | 122I | 122Xe | 122Cs | 122Ba | 122La | 122Ce | 122Pr | |||||||||||||||
| 123 | 123Ru | 123Rh | 123Pd | 123Ag | 123Cd | 123In | 123Sn | 123Sb | 123Te | 123I | 123Xe | 123Cs | 123Ba | 123La | 123Ce | 123Pr | |||||||||||||||
| 124 | 124Ru | 124Rh | 124Pd | 124Ag | 124Cd | 124In | 124Sn | 124Sb | 124Te | 124I | 124Xe | 124Cs | 124Ba | 124La | 124Ce | 124Pr | 124Nd | ||||||||||||||
| 125 | 125Rh | 125Pd | 125Ag | 125Cd | 125In | 125Sn | 125Sb | 125Te | 125I | 125Xe | 125Cs | 125Ba | 125La | 125Ce | 125Pr | 125Nd | |||||||||||||||
| 126 | 126Rh | 126Pd | 126Ag | 126Cd | 126In | 126Sn | 126Sb | 126Te | 126I | 126Xe | 126Cs | 126Ba | 126La | 126Ce | 126Pr | 126Nd | 126Pm | ||||||||||||||
| 127 | 127Pd | 127Ag | 127Cd | 127In | 127Sn | 127Sb | 127Te | 127I | 127Xe | 127Cs | 127Ba | 127La | 127Ce | 127Pr | 127Nd | 127Pm | |||||||||||||||
| 128 | 128Pd | 128Ag | 128Cd | 128In | 128Sn | 128Sb | 128Te | 128I | 128Xe | 128Cs | 128Ba | 128La | 128Ce | 128Pr | 128Nd | 128Pm | 128Sm | ||||||||||||||
| 129 | 129Ag | 129Cd | 129In | 129Sn | 129Sb | 129Te | 129I | 129Xe | 129Cs | 129Ba | 129La | 129Ce | 129Pr | 129Nd | 129Pm | 129Sm | |||||||||||||||
| 130 | 130Ag | 130Cd | 130In | 130Sn | 130Sb | 130Te | 130I | 130Xe | 130Cs | 130Ba | 130La | 130Ce | 130Pr | 130Nd | 130Pm | 130Sm | 130Eu | ||||||||||||||
| 131 | 131Cd | 131In | 131Sn | 131Sb | 131Te | 131I | 131Xe | 131Cs | 131Ba | 131La | 131Ce | 131Pr | 131Nd | 131Pm | 131Sm | 131Eu | |||||||||||||||
| 132 | 132Cd | 132In | 132Sn | 132Sb | 132Te | 132I | 132Xe | 132Cs | 132Ba | 132La | 132Ce | 132Pr | 132Nd | 132Pm | 132Sm | 132Eu | |||||||||||||||
| 133 | 133Cd | 133In | 133Sn | 133Sb | 133Te | 133I | 133Xe | 133Cs | 133Ba | 133La | 133Ce | 133Pr | 133Nd | 133Pm | 133Sm | 133Eu | |||||||||||||||
| 134 | 134In | 134Sn | 134Sb | 134Te | 134I | 134Xe | 134Cs | 134Ba | 134La | 134Ce | 134Pr | 134Nd | 134Pm | 134Sm | 134Eu | 134Gd | |||||||||||||||
| 135 | 135In | 135Sn | 135Sb | 135Te | 135I | 135Xe | 135Cs | 135Ba | 135La | 135Ce | 135Pr | 135Nd | 135Pm | 135Sm | 135Eu | 135Gd | 135Tb | ||||||||||||||
| 136 | 136Sn | 136Sb | 136Te | 136I | 136Xe | 136Cs | 136Ba | 136La | 136Ce | 136Pr | 136Nd | 136Pm | 136Sm | 136Eu | 136Gd | 136Tb | |||||||||||||||
| 137 | 137Sn | 137Sb | 137Te | 137I | 137Xe | 137Cs | 137Ba | 137La | 137Ce | 137Pr | 137Nd | 137Pm | 137Sm | 137Eu | 137Gd | 137Tb | |||||||||||||||
| 138 | 138Sn | 138Sb | 138Te | 138I | 138Xe | 138Cs | 138Ba | 138La | 138Ce | 138Pr | 138Nd | 138Pm | 138Sm | 138Eu | 138Gd | 138Tb | 138Dy | ||||||||||||||
| 139 | 139Sb | 139Te | 139I | 139Xe | 139Cs | 139Ba | 139La | 139Ce | 139Pr | 139Nd | 139Pm | 139Sm | 139Eu | 139Gd | 139Tb | 139Dy | |||||||||||||||
| 140 | 140Sb | 140Te | 140I | 140Xe | 140Cs | 140Ba | 140La | 140Ce | 140Pr | 140Nd | 140Pm | 140Sm | 140Eu | 140Gd | 140Tb | 140Dy | 140Ho | ||||||||||||||
| 141 | 141Te | 141I | 141Xe | 141Cs | 141Ba | 141La | 141Ce | 141Pr | 141Nd | 141Pm | 141Sm | 141Eu | 141Gd | 141Tb | 141Dy | 141Ho | |||||||||||||||
| 142 | 142Te | 142I | 142Xe | 142Cs | 142Ba | 142La | 142Ce | 142Pr | 142Nd | 142Pm | 142Sm | 142Eu | 142Gd | 142Tb | 142Dy | 142Ho | |||||||||||||||
| 143 | 143Te | 143I | 143Xe | 143Cs | 143Ba | 143La | 143Ce | 143Pr | 143Nd | 143Pm | 143Sm | 143Eu | 143Gd | 143Tb | 143Dy | 143Ho | 143Er |
Kernisotone Nuklide des Tellurs
Die zu den Tellur-Kernen isotonen Nuklide befinden sich in der jeweiligen Tabellenzeile; N = Anzahl der Neutronen.
| 53 | 54 | 55 | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | 85 | 86 | 87 | 88 | 89 | 90 | 91 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 82Cu | ||||||||||||||||||||||||||||||||||||||
| 83Zn | 84Zn | |||||||||||||||||||||||||||||||||||||
| 84Ga | 85Ga | 86Ga | 87Ga | |||||||||||||||||||||||||||||||||||
| 85Ge | 86Ge | 87Ge | 88Ge | 89Ge | ||||||||||||||||||||||||||||||||||
| 86As | 87As | 88As | 89As | 90As | 91As | 92As | ||||||||||||||||||||||||||||||||
| 87Se | 88Se | 89Se | 90Se | 91Se | 92Se | 93Se | 94Se | |||||||||||||||||||||||||||||||
| 88Br | 89Br | 90Br | 91Br | 92Br | 93Br | 94Br | 95Br | 96Br | 97Br | 98Br | ||||||||||||||||||||||||||||
| 89Kr | 90Kr | 91Kr | 92Kr | 93Kr | 94Kr | 95Kr | 96Kr | 97Kr | 98Kr | 99Kr | 100Kr | 101Kr | ||||||||||||||||||||||||||
| 90Rb | 91Rb | 92Rb | 93Rb | 94Rb | 95Rb | 96Rb | 97Rb | 98Rb | 99Rb | 100Rb | 101Rb | 102Rb | ||||||||||||||||||||||||||
| 91Sr | 92Sr | 93Sr | 94Sr | 95Sr | 96Sr | 97Sr | 98Sr | 99Sr | 100Sr | 101Sr | 102Sr | 103Sr | 104Sr | 105Sr | 106Sr | 107Sr | ||||||||||||||||||||||
| 92Y | 93Y | 94Y | 95Y | 96Y | 97Y | 98Y | 99Y | 100Y | 101Y | 102Y | 103Y | 104Y | 105Y | 106Y | 107Y | 108Y | 109Y | |||||||||||||||||||||
| 93Zr | 94Zr | 95Zr | 96Zr | 97Zr | 98Zr | 99Zr | 100Zr | 101Zr | 102Zr | 103Zr | 104Zr | 105Zr | 106Zr | 107Zr | 108Zr | 109Zr | 110Zr | 111Zr | 112Zr | |||||||||||||||||||
| 94Nb | 95Nb | 96Nb | 97Nb | 98Nb | 99Nb | 100Nb | 101Nb | 102Nb | 103Nb | 104Nb | 105Nb | 106Nb | 107Nb | 108Nb | 109Nb | 110Nb | 111Nb | 112Nb | 113Nb | 114Nb | 115Nb | |||||||||||||||||
| 95Mo | 96Mo | 97Mo | 98Mo | 99Mo | 100Mo | 101Mo | 102Mo | 103Mo | 104Mo | 105Mo | 106Mo | 107Mo | 108Mo | 109Mo | 110Mo | 111Mo | 112Mo | 113Mo | 114Mo | 115Mo | 116Mo | 117Mo | ||||||||||||||||
| 96Tc | 97Tc | 98Tc | 99Tc | 100Tc | 101Tc | 102Tc | 103Tc | 104Tc | 105Tc | 106Tc | 107Tc | 108Tc | 109Tc | 110Tc | 111Tc | 112Tc | 113Tc | 114Tc | 115Tc | 116Tc | 117Tc | 118Tc | 119Tc | 120Tc | ||||||||||||||
| 97Ru | 98Ru | 99Ru | 100Ru | 101Ru | 102Ru | 103Ru | 104Ru | 105Ru | 106Ru | 107Ru | 108Ru | 109Ru | 110Ru | 111Ru | 112Ru | 113Ru | 114Ru | 115Ru | 116Ru | 117Ru | 118Ru | 119Ru | 120Ru | 121Ru | 122Ru | 123Ru | 124Ru | |||||||||||
| 98Rh | 99Rh | 100Rh | 101Rh | 102Rh | 103Rh | 104Rh | 105Rh | 106Rh | 107Rh | 108Rh | 109Rh | 110Rh | 111Rh | 112Rh | 113Rh | 114Rh | 115Rh | 116Rh | 117Rh | 118Rh | 119Rh | 120Rh | 121Rh | 122Rh | 123Rh | 124Rh | 125Rh | 126Rh | ||||||||||
| 99Pd | 100Pd | 101Pd | 102Pd | 103Pd | 104Pd | 105Pd | 106Pd | 107Pd | 108Pd | 109Pd | 110Pd | 111Pd | 112Pd | 113Pd | 114Pd | 115Pd | 116Pd | 117Pd | 118Pd | 119Pd | 120Pd | 121Pd | 122Pd | 123Pd | 124Pd | 125Pd | 126Pd | 127Pd | 128Pd | |||||||||
| 100Ag | 101Ag | 102Ag | 103Ag | 104Ag | 105Ag | 106Ag | 107Ag | 108Ag | 109Ag | 110Ag | 111Ag | 112Ag | 113Ag | 114Ag | 115Ag | 116Ag | 117Ag | 118Ag | 119Ag | 120Ag | 121Ag | 122Ag | 123Ag | 124Ag | 125Ag | 126Ag | 127Ag | 128Ag | 129Ag | 130Ag | ||||||||
| 101Cd | 102Cd | 103Cd | 104Cd | 105Cd | 106Cd | 107Cd | 108Cd | 109Cd | 110Cd | 111Cd | 112Cd | 113Cd | 114Cd | 115Cd | 116Cd | 117Cd | 118Cd | 119Cd | 120Cd | 121Cd | 122Cd | 123Cd | 124Cd | 125Cd | 126Cd | 127Cd | 128Cd | 129Cd | 130Cd | 131Cd | 132Cd | 133Cd | ||||||
| 102In | 103In | 104In | 105In | 106In | 107In | 108In | 109In | 110In | 111In | 112In | 113In | 114In | 115In | 116In | 117In | 118In | 119In | 120In | 121In | 122In | 123In | 124In | 125In | 126In | 127In | 128In | 129In | 130In | 131In | 132In | 133In | 134In | 135In | |||||
| 103Sn | 104Sn | 105Sn | 106Sn | 107Sn | 108Sn | 109Sn | 110Sn | 111Sn | 112Sn | 113Sn | 114Sn | 115Sn | 116Sn | 117Sn | 118Sn | 119Sn | 120Sn | 121Sn | 122Sn | 123Sn | 124Sn | 125Sn | 126Sn | 127Sn | 128Sn | 129Sn | 130Sn | 131Sn | 132Sn | 133Sn | 134Sn | 135Sn | 136Sn | 137Sn | 138Sn | |||
| 104Sb | 105Sb | 106Sb | 107Sb | 108Sb | 109Sb | 110Sb | 111Sb | 112Sb | 113Sb | 114Sb | 115Sb | 116Sb | 117Sb | 118Sb | 119Sb | 120Sb | 121Sb | 122Sb | 123Sb | 124Sb | 125Sb | 126Sb | 127Sb | 128Sb | 129Sb | 130Sb | 131Sb | 132Sb | 133Sb | 134Sb | 135Sb | 136Sb | 137Sb | 138Sb | 139Sb | 140Sb | ||
| 105Te | 106Te | 107Te | 108Te | 109Te | 110Te | 111Te | 112Te | 113Te | 114Te | 115Te | 116Te | 117Te | 118Te | 119Te | 120Te | 121Te | 122Te | 123Te | 124Te | 125Te | 126Te | 127Te | 128Te | 129Te | 130Te | 131Te | 132Te | 133Te | 134Te | 135Te | 136Te | 137Te | 138Te | 139Te | 140Te | 141Te | 142Te | 143Te |
| 108I | 109I | 110I | 111I | 112I | 113I | 114I | 115I | 116I | 117I | 118I | 119I | 120I | 121I | 122I | 123I | 124I | 125I | 126I | 127I | 128I | 129I | 130I | 131I | 132I | 133I | 134I | 135I | 136I | 137I | 138I | 139I | 140I | 141I | 142I | 143I | 144I | ||
| 109Xe | 110Xe | 111Xe | 112Xe | 113Xe | 114Xe | 115Xe | 116Xe | 117Xe | 118Xe | 119Xe | 120Xe | 121Xe | 122Xe | 123Xe | 124Xe | 125Xe | 126Xe | 127Xe | 128Xe | 129Xe | 130Xe | 131Xe | 132Xe | 133Xe | 134Xe | 135Xe | 136Xe | 137Xe | 138Xe | 139Xe | 140Xe | 141Xe | 142Xe | 143Xe | 144Xe | 145Xe | ||
| 112Cs | 113Cs | 114Cs | 115Cs | 116Cs | 117Cs | 118Cs | 119Cs | 120Cs | 121Cs | 122Cs | 123Cs | 124Cs | 125Cs | 126Cs | 127Cs | 128Cs | 129Cs | 130Cs | 131Cs | 132Cs | 133Cs | 134Cs | 135Cs | 136Cs | 137Cs | 138Cs | 139Cs | 140Cs | 141Cs | 142Cs | 143Cs | 144Cs | 145Cs | 146Cs | ||||
| 114Ba | 115Ba | 116Ba | 117Ba | 118Ba | 119Ba | 120Ba | 121Ba | 122Ba | 123Ba | 124Ba | 125Ba | 126Ba | 127Ba | 128Ba | 129Ba | 130Ba | 131Ba | 132Ba | 133Ba | 134Ba | 135Ba | 136Ba | 137Ba | 138Ba | 139Ba | 140Ba | 141Ba | 142Ba | 143Ba | 144Ba | 145Ba | 146Ba | 147Ba | |||||
| 116La | 117La | 118La | 119La | 120La | 121La | 122La | 123La | 124La | 125La | 126La | 127La | 128La | 129La | 130La | 131La | 132La | 133La | 134La | 135La | 136La | 137La | 138La | 139La | 140La | 141La | 142La | 143La | 144La | 145La | 146La | 147La | 148La | ||||||
| 119Ce | 120Ce | 121Ce | 122Ce | 123Ce | 124Ce | 125Ce | 126Ce | 127Ce | 128Ce | 129Ce | 130Ce | 131Ce | 132Ce | 133Ce | 134Ce | 135Ce | 136Ce | 137Ce | 138Ce | 139Ce | 140Ce | 141Ce | 142Ce | 143Ce | 144Ce | 145Ce | 146Ce | 147Ce | 148Ce | 149Ce | ||||||||
| 121Pr | 122Pr | 123Pr | 124Pr | 125Pr | 126Pr | 127Pr | 128Pr | 129Pr | 130Pr | 131Pr | 132Pr | 133Pr | 134Pr | 135Pr | 136Pr | 137Pr | 138Pr | 139Pr | 140Pr | 141Pr | 142Pr | 143Pr | 144Pr | 145Pr | 146Pr | 147Pr | 148Pr | 149Pr | 150Pr | |||||||||
| 124Nd | 125Nd | 126Nd | 127Nd | 128Nd | 129Nd | 130Nd | 131Nd | 132Nd | 133Nd | 134Nd | 135Nd | 136Nd | 137Nd | 138Nd | 139Nd | 140Nd | 141Nd | 142Nd | 143Nd | 144Nd | 145Nd | 146Nd | 147Nd | 148Nd | 149Nd | 150Nd | 151Nd | |||||||||||
| 126Pm | 127Pm | 128Pm | 129Pm | 130Pm | 131Pm | 132Pm | 133Pm | 134Pm | 135Pm | 136Pm | 137Pm | 138Pm | 139Pm | 140Pm | 141Pm | 142Pm | 143Pm | 144Pm | 145Pm | 146Pm | 147Pm | 148Pm | 149Pm | 150Pm | 151Pm | 152Pm | ||||||||||||
| 128Sm | 129Sm | 130Sm | 131Sm | 132Sm | 133Sm | 134Sm | 135Sm | 136Sm | 137Sm | 138Sm | 139Sm | 140Sm | 141Sm | 142Sm | 143Sm | 144Sm | 145Sm | 146Sm | 147Sm | 148Sm | 149Sm | 150Sm | 151Sm | 152Sm | 153Sm | |||||||||||||
| 130Eu | 131Eu | 132Eu | 133Eu | 134Eu | 135Eu | 136Eu | 137Eu | 138Eu | 139Eu | 140Eu | 141Eu | 142Eu | 143Eu | 144Eu | 145Eu | 146Eu | 147Eu | 148Eu | 149Eu | 150Eu | 151Eu | 152Eu | 153Eu | 154Eu | ||||||||||||||
| 134Gd | 135Gd | 136Gd | 137Gd | 138Gd | 139Gd | 140Gd | 141Gd | 142Gd | 143Gd | 144Gd | 145Gd | 146Gd | 147Gd | 148Gd | 149Gd | 150Gd | 151Gd | 152Gd | 153Gd | 154Gd | 155Gd | |||||||||||||||||
| 135Tb | 136Tb | 137Tb | 138Tb | 139Tb | 140Tb | 141Tb | 142Tb | 143Tb | 144Tb | 145Tb | 146Tb | 147Tb | 148Tb | 149Tb | 150Tb | 151Tb | 152Tb | 153Tb | 154Tb | 155Tb | 156Tb | |||||||||||||||||
| 138Dy | 139Dy | 140Dy | 141Dy | 142Dy | 143Dy | 144Dy | 145Dy | 146Dy | 147Dy | 148Dy | 149Dy | 150Dy | 151Dy | 152Dy | 153Dy | 154Dy | 155Dy | 156Dy | 157Dy | |||||||||||||||||||
| 140Ho | 141Ho | 142Ho | 143Ho | 144Ho | 145Ho | 146Ho | 147Ho | 148Ho | 149Ho | 150Ho | 151Ho | 152Ho | 153Ho | 154Ho | 155Ho | 156Ho | 157Ho | 158Ho | ||||||||||||||||||||
| 143Er | 144Er | 145Er | 146Er | 147Er | 148Er | 149Er | 150Er | 151Er | 152Er | 153Er | 154Er | 155Er | 156Er | 157Er | 158Er | 159Er | ||||||||||||||||||||||
| 144Tm | 145Tm | 146Tm | 147Tm | 148Tm | 149Tm | 150Tm | 151Tm | 152Tm | 153Tm | 154Tm | 155Tm | 156Tm | 157Tm | 158Tm | 159Tm | 160Tm | ||||||||||||||||||||||
| 148Yb | 149Yb | 150Yb | 151Yb | 152Yb | 153Yb | 154Yb | 155Yb | 156Yb | 157Yb | 158Yb | 159Yb | 160Yb | 161Yb | |||||||||||||||||||||||||
| 150Lu | 151Lu | 152Lu | 153Lu | 154Lu | 155Lu | 156Lu | 157Lu | 158Lu | 159Lu | 160Lu | 161Lu | 162Lu | ||||||||||||||||||||||||||
| 153Hf | 154Hf | 155Hf | 156Hf | 157Hf | 158Hf | 159Hf | 160Hf | 161Hf | 162Hf | 163Hf | ||||||||||||||||||||||||||||
| 155Ta | 156Ta | 157Ta | 158Ta | 159Ta | 160Ta | 161Ta | 162Ta | 163Ta | 164Ta | |||||||||||||||||||||||||||||
| 158W | 159W | 160W | 161W | 162W | 163W | 164W | 165W | |||||||||||||||||||||||||||||||
| 159Re | 160Re | 161Re | 162Re | 163Re | 164Re | 165Re | 166Re | |||||||||||||||||||||||||||||||
| 161Os | 162Os | 163Os | 164Os | 165Os | 166Os | 167Os | ||||||||||||||||||||||||||||||||
| 164Ir | 165Ir | 166Ir | 167Ir | 168Ir | ||||||||||||||||||||||||||||||||||
| 166Pt | 167Pt | 168Pt | 169Pt | |||||||||||||||||||||||||||||||||||
| 170Au | ||||||||||||||||||||||||||||||||||||||
| 171Hg |
Literatur und Hinweise
Eigenschaften der Tellur-Isotope
[1] - NuDat: National Nuclear Data Center, Brookhaven National Laboratory, based on ENSDF and the Nuclear Wallet Cards.
[2] - G. Audi et. al.: The NUBASE evaluation of nuclear and decay properties. Nuclear Physics, (2003), DOI 10.1016/j.nuclphysa.2003.11.001.
[3] - Live Chart of Nuclides. Nuclear structure and decay data.
Tellur: Kernmagnetische Eigenschaften - 123Te-NMR, 125Te-NMR
[4] - N. J. Stone: Table of nuclear magnetic dipole and electric quadrupole moments. Atomic Data and Nuclear Data Tables, (2005), DOI 10.1016/j.adt.2005.04.001.
[5] - Pekka Pyykkö: Year-2008 nuclear quadrupole moments. Molecular Physics, (2008), DOI 10.1080/00268970802018367.
[6] - Pekka Pyykkö: Year-2017 nuclear quadrupole moments. Molecular Physics, (2018), DOI 10.1080/00268976.2018.1426131.
[7] - N. J. Stone: Table of recommended nuclear magnetic dipole moments. IAEA, (2019).
Weitere Quellen:
[8] - Isotopenhäufigkeiten, Atommassen und Isotopenmassen: Siehe unter dem jeweiligen Stichwort.
[9] - A. Alessandrello et al.:
New limits on naturally occurring electron capture of 123Te.
In: Physical Review C, (2003), DOI 10.1103/PhysRevC.67.014323.
[10] - J. Kathawa, C. Fry, M. Thoennessen:
Discovery of palladium, antimony, tellurium, iodine, and xenon isotopes.
In: Atomic Data and Nuclear Data Tables, (2013), DOI 10.1016/j.adt.2012.01.004.
Kategorie: Chemische Elemente
Letzte Änderung am 12.12.2022.
Permalink: https://www.internetchemie.info/chemische-elemente/tellur-isotope.php.
© 1996 - 2026 Internetchemie ChemLin